Abstract
Multivesicular bodies (MVBs) are spherical endosomal organelles containing small vesicles formed by inward budding of the limiting membrane into the endosomal lumen. In mammalian red cells and cells of immune system, MVBs fuse with the plasma membrane in an exocytic manner, leading to release their contents including internal vesicles into the extracellular space. These released vesicles are termed exosomes. Transmission electron microscopy studies have shown that paramural vesicles situated between the plasma membrane and the cell wall occur in various cell wall-associated processes and are similar to exosomes both in location and in morphology. Our recent studies have revealed that MVBs and paramural vesicles proliferate when cell wall appositions are rapidly deposited beneath fungal penetration attempts or during plugging of plasmodesmata between hypersensitive cells and their intact neighboring cells. This indicates a potential secretion of exosome-like vesicles into the extracellular space by fusion of MVBs with the plasma membrane. This MVB-mediated secretion pathway was proposed on the basis of pioneer studies of MVBs and paramural vesicles in plants some forty years ago. Here, we recall the attention to the occurrence of MVB-mediated secretion of exosomes in plants.
Key Words: cell wall, endocytosis, endosome, exocytosis, exosome, multivesicular body, paramural body
Multivesicular bodies (MVBs) are spherical endosomal organelles containing a number of small vesicles formed by inward budding of the limiting membrane into the endosomal lumen.1 MVBs contain endocytosed cargoes and deliver them into lysosomal/vacuolar compartments for degradation. They also incorporate newly synthesized proteins destined for lysosomal/vacuolar compartments.2 In mammalian cells of hematopoietic origin, endosomal MVBs function in removal of endocytosed surface proteins in an exocytic manner. They are redirected to the plasma membrane, where they release their contents including internal vesicles into the extracellular space by membrane fusion. The released vesicles are termed exosomes.3 During reticulocyte maturation to erythrocyte, a group of surface proteins, such as the transferrin receptor, become obsolete and are discarded via MVB-mediated secretion.3 Time-course transmission electron microscopy (TEM) first revealed that colloidal gold-transferrin was internalized into MVBs via receptor-mediated endocytosis and then transferrin together with its receptor were delivered into the extracellular space via the fusion of MVBs with the plasma membrane of reticulocytes.4 Some other cell types of hematopoietic origin, such as activated platelets, cytotoxic T cells and antigen-presenting cells, also secrete exosomes. Exosomes thus may play a role in various physiological processes other than discarding obsolete proteins.3
Our recent TEM studies provided ultrastructural evidence on the enhanced vesicle trafficking in barley leaf cells attacked by the biotrophic powdery mildew fungus. Multivesicular compartments including MVBs, intravacuolar MVBs, and paramural bodies turned out to proliferate in intact host cells during formation of cell wall appositions (papilla response), in the hypersensitive response, and during accommodation of haustoria.5,6 MVBs proliferated in the cytoplasm of haustorium-containing epidermal cells during compatible interactions and near sites of cell wall-associated oxidative microburst either during the papilla response or during the hypersensitive response. Because MVBs in plant cells have been demonstrated to be endosomal compartments,7–9 they may participate in internalization of nutrients from the apoplast of intact haustorium-containing epidermal cells and sequestration of damaged membranes and deleterious materials originating from the oxidative microburst.5,6 The presence of intravacuolar MVBs with double limiting membranes (Fig. 1A) indicates an engulfment of MVBs by the tonoplast and a vacuole-mediated autophagy of MVBs.5,6 MVBs, as prevacuolar compartments in plant cells,9 thus probably deliver their contents into the central vacuole via both the fusion with the tonoplast and the engulfment by the tonoplast (Fig. 2A and B). On the other hand, paramural bodies, in which small vesicles are situated between the cell wall and the plasma membrane, were associated with cell wall appositions deposited beneath fungal penetration attempts (Fig. 1B) or around hypersensitive cells including sites of plugged plasmodesmata (Fig. 1C and D).5,6 Because paramural vesicles are similar to exosomes both in location and in morphology, we speculated that MVBs fuse with the plasma membrane in an exocytic manner to form paramural bodies.5,6 Endocytosed cell surface materials in endosomal MVBs may be reused and delivered together with newly synthesized materials in Golgi apparatus-derived vesicles to cell wall appositions, which are deposited rapidly to prevent fungal penetration (Fig. 2A) or to contain hypersensitive cell death (Fig. 2B). MVBs thus may be driven along two distinct pathways to deliver their contents into either central vacuole or extracellular space.
Figure 1.
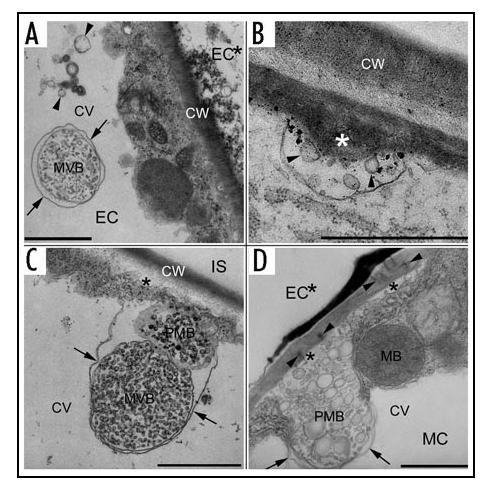
Multivesicular compartments in intact cells in barley leaves attacked by the barley powdery mildew fungus. (A) An intravacuolar multivesicular body (MVB) with double limiting membranes in an intact epidermal cell (EC) adjacent to a hypersensitive epidermal cell (EC*). The arrows point to the outer limiting membrane, which is seemingly derived from the tonoplast. Note that neighboring intravacuolar vesicles (in between two arrowheads) may result from degradation of double limiting membranes of intravacuolar MVBs or may be delivered into the vacuole by MVB-fusion with the tonoplast. (B) Paramural vesicles (arrowheads) in a paramural body associated with cell wall appositions (asterisk) deposited by an intact epidermal cell. (C) A multivesicular body (MVB) in contact with a paramural body (PMB) (a nonmedian section) associated with cell wall appositions (asterisk) deposited by an intact mesophyll cell adjacent to a hypersensitive mesophyll cell. Note that cell wall appositions deposit beside an intercellular space (IS). The arrows point to the tonoplast. (D) A paramural body (PMB) associated with cell wall appositions (asterisks) blocking plasmodesmata (in between two arrowheads) at the side of an intact mesophyll cell (MC) underlying a hypersensitive epidermal cell (EC*). The arrows point to the tonoplast. CV, central vacuole; CW, cell wall; MB, microbody. Bars, 1µm.
Figure 2.
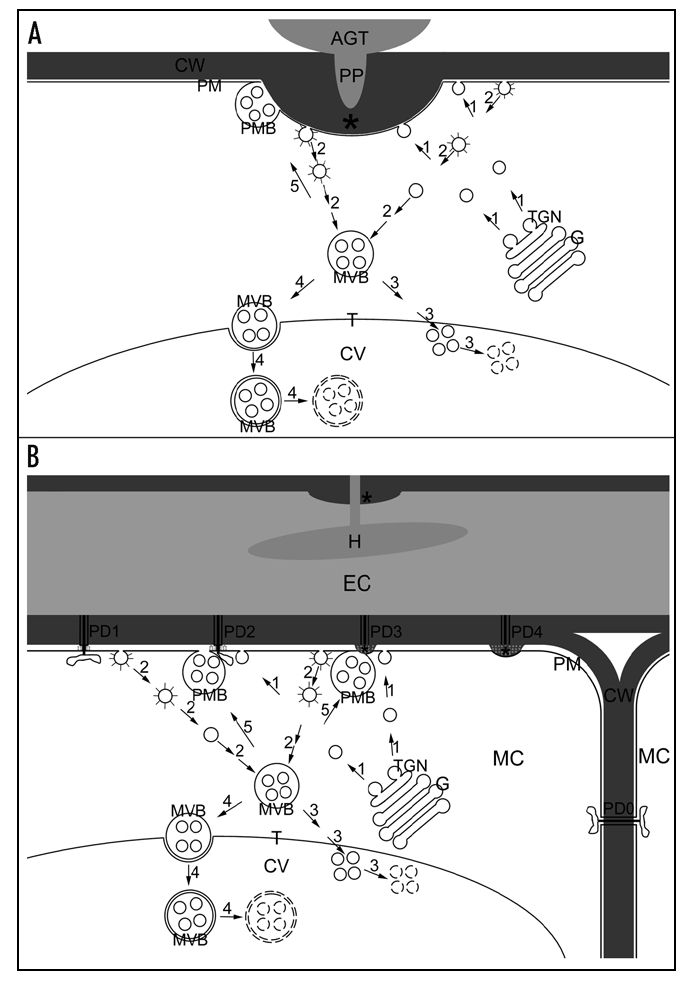
Hypothetical diagram of delivery of endocytosed cell surface materials via MVBs into the central vacuole or the extracellular space where intact barley cells deposit cell wall appositions. (A) Deposition of cell wall appositions (asterisk) beneath powdery mildew penetration attempts. AGT, appressorial germ tube; PP, penetration peg. (B) Deposition of cell wall appositions (asterisks) against constricted plasmodesmata (PD) between a hypersensitive epidermal cell (EC) penetrated by the powdery mildew fungus and an underlying mesophyll cell (MC). H, haustorium. Arrows and numbers show pathways of vesicle trafficking. 1, Secretion of Golgi-derived vesicles containing newly synthesized materials; G, Golgi body; TGN, trans-Golgi network; 2, Endocytosis of cell surface materials from coated pits (coated open circles) via coated vesicles (coated circles) to multivesicular bodies (MVB); 3, Delivery of endocytosed materials for degradation inside the central vacuole (CV) via membrane fusion between MVBs and the tonoplast (T); small broken circles, vesicles in degradation; 4, Delivery of endocytosed materials for degradation inside the central vacuole via engulfment of MVBs by the tonoplast; large broken circles; MVB limiting membranes in degradation; 5, delivery of endocytosed materials into the extracellular space for deposition of cell wall appositions (asterisks) via membrane fusion between MVBs and the plasma membrane (PM). CW, cell wall; PMB, paramural body. PD0, 1, 2, 3 and 4 represent stages of plugging plasmodesmata. PD0, open plasmodesmata between two intact mesophyll cells (MC) subjacent to the hypersensitive epidermal cell (EC); PD1, constriction of plasmodesmata by callose (grey dots) deposition at plasmodesmal neck region; PD2, constricted plasmodesmata associated with plasmodesma-targeted secretion; PD3, further blocking of plasmodesmata by deposition of cell wall appositions; PD4, completely blocked plasmodesmata.
Earlier than the discovery in animal cell systems,4 it was proposed in two independent papers in 1967 that the fusion of MVBs with the plasma membrane might result in the release of small vesicles into the extracellular space in fungi and in higher plants.10,11 Several lines of evidence support the occurrence of MVB-mediated secretion of exosome-like vesicles in plants. First, vesicles of the same morphology as MVB internal vesicles have been observed in extracellular spaces or paramural spaces in various types of plant cells in various plant species by TEM.12 An early study on endocytosis by soybean protoplasts also showed small extracellular vesicles attaching on the plasma membrane.8 Second, cooccurrence of MVBs and paramural vesicles has been observed in processes of cell proliferation, cell differentiation, and cell response to abiotic and biotic stress. Examples are cell plate formation,13,14 secondary wall thickening,15,16 cold hardness,17,18 and deposition of cell wall appositions upon pathogen attack.5,6,19–21 Third, identical molecular components, such as arabinogalactan proteins22,23 and peroxidases,6 have been immunolocalized in both MVBs and paramural bodies. Despite these pieces of evidence, a conclusive demonstration of MVB-mediated secretion of exosomes in plants requires further exploration.
The presently available experimental systems, approaches, and membrane markers may allow future demonstration of MVB-mediated secretion of exosomes in plants. Recent in vivo real-time observation and colocalization of cell surface and endosomal markers have already revealed that endosomes filled with endocytosed preexisting cell wall and plasma membrane materials are rapidly delivered to cytokinetic spaces to form cell plates in dividing tobacco, Arabidopsis, and maize cells.24 Because TEM observed paramural bodies attaching to cell plates13 and MVBs in the vicinity of cell plates during all stages of cell plate formation,14,25,26 MVBs and paramural bodies may participate in delivery of endocytosed building blocks to cell plates. Jiang's and Robinson's labs together developed a transgenic tobacco BY-2 cell line stably expressing a YFP-labeled vacuolar sorting receptor protein and antibodies against the vacuolar sorting receptor protein localized to the limiting membrane of MVBs.9 These tools together with live cell imaging and immunoelectron microscopy may allow visualization of MVB-fusion to the new plasma membrane, of vacuolar sorting receptors in both the limiting membrane of MVBs and the new plasma membrane, and of identical cell plate components in both internal vesicles of MVBs and paramural vesicles.
In spite of obvious differences in plant and animal cytokinesis, the generation of cell plates by cell-plate-directed fusion of endosomes resembles the plugging of midbody canals by midbody-directed endosomes to separate daughter cells at the terminal phase of animal cytokinesis.27 Likely, functional similarities of the fusion between endosomal MVBs and the plasma membrane to eliminate unwanted cell contents may also exist in maturation of mammalian red blood cells and plant sieve elements in the sense that the fusion of MVBs with the plasma membrane may occur during maturation of the latter.28 On the other hand, although plant cells may secrete MVB-derived exosomes in defense response upon pathogen attack,5,6 plant cell walls rule out the direct intercellular communication during the immune response mediated by exosomes in the circulation of mammals.3 In contrast, plasmodesma-directed secretion of exosomes would block the cell-to-cell communication between hypersensitive cells and their neighboring cells during hypersensitive response.5 Further exploration will lead us to a better understanding of similarities and differences of exosome secretion between plants and animals.
Acknowledgements
This work was supported by grants BE 1925/7-1 and 1925/7-2 from the German Research Foundation.
Abbreviations
- MVB
multivesicular body
- TEM
transmission electron microscopy
- YFP
yellow, fluorescent protein
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3596
References
- 1.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 2.Geldner N. The plant endosomal system-its structure and role in signal transduction and plant development. Planta. 2004;219:547–560. doi: 10.1007/s00425-004-1302-x. [DOI] [PubMed] [Google Scholar]
- 3.Denzer K, Kleijmeer MJ, Heijnen HFG, Stoorvogel W, Geuze HJ. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 4.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An Q, Ehlers K, Kogel KH, van Bel AJE, Hückelhoven R. Multivesicular compartments proliferate in susceptible and resistant MLA12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol. 2006;172:563–576. doi: 10.1111/j.1469-8137.2006.01844.x. [DOI] [PubMed] [Google Scholar]
- 6.An Q, Hückelhoven R, Kogel KH, van Bel AJE. Multivesicular bodies participate in a cell wall associated defense response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol. 2006;8:1009–1019. doi: 10.1111/j.1462-5822.2006.00683.x. [DOI] [PubMed] [Google Scholar]
- 7.Tanchak MA, Griffing LR, Mersey BG, Fowke LC. Endocytosis of cationized ferritin by coated vesicles of soybean protoplasts. Planta. 1984;162:481–486. doi: 10.1007/BF00399912. [DOI] [PubMed] [Google Scholar]
- 8.Tanchak MA, Fowke LC. The morphology of multivesicular bodies in soybean protoplasts and their role in endocytosis. Protoplasma. 1987;138:173–182. [Google Scholar]
- 9.Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell. 2004;16:672–693. doi: 10.1105/tpc.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchant R, Peat A, Banbury GH. The ultrastructural basis of hyphal growth. New Phytol. 1967;66:623–629. [Google Scholar]
- 11.Halperin W, Jensen WA. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J Ultrastruct Res. 1967;18:428–443. doi: 10.1016/s0022-5320(67)80128-x. [DOI] [PubMed] [Google Scholar]
- 12.Marchant R, Robards AW. Membrane systems associated with the plasmalemma of plant cells. Ann Bot. 1968;32:457–471. [Google Scholar]
- 13.Lehmann H, Schulz D. Elektronenmikroskopische Untersuchungen von Differenzierungsv orgängen bei Moosen II. Die Zellplatten- und Zellwandbildung. Planta. 1969;85:313–325. doi: 10.1007/BF00381280. (Ger). [DOI] [PubMed] [Google Scholar]
- 14.Samuels AL, Giddings TH, Jr, Staehelin LA. Cytokinesis in tobacco BY-2 and root tip cells: A new model of cell plate formation in higher plants. J Cell Biol. 1995;130:1345–1357. doi: 10.1083/jcb.130.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chafe SC. Cell wall formation and “protective layer” development in the xylem parenchyma of trembling aspen. Protoplasma. 1974;80:335–354. doi: 10.1007/BF02055774. [DOI] [PubMed] [Google Scholar]
- 16.Robards AW, Kidwai P. Vesicular involvement in differentiating plant vascular cells. New Phytol. 1969;68:343–349. [Google Scholar]
- 17.Niki T, Sakai A. Ultrastructural changes related to frost hardiness in the cortical parenchyma cells from mulberry twigs. Plant Cell Physiol. 1981;22:171–183. [Google Scholar]
- 18.Wisniewski M, Ashworth EN. A comparison of seasonal ultrastructural changes in stem tissues of peach (Prunus persica) that exhibit contrasting mechanisms of cold hardiness. Bot Gaz. 1986;147:407–417. [Google Scholar]
- 19.Bestwick CS, Bennett MH, Mansfield JW. Hrp mutant of Pseudomonas syringae pv phaseolicola induces cell wall alterations but not membrane damage leading to the hypersensitive reaction in lettuce. Plant Physiol. 1995;108:503–516. doi: 10.1104/pp.108.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allison AV, Shalla TA. The ultrastructure of local lesions induced by potato virus X: A sequence of cytological events in the course of infection. Phytopathology. 1974;64:784–793. [Google Scholar]
- 21.McMullen CR, Gardner WS, Myers GA. Ultrastructure of cell-wall thickenings and paramural bodies induced by barley stripe mosaic virus. Phytopathology. 1977;67:462–467. [Google Scholar]
- 22.Herman EM, Lamb CJ. Arabinogalactan-rich glycoproteins are localized on the cell surface and in intravacuolar multivesicular bodies. Plant Physiol. 1992;98:264–272. doi: 10.1104/pp.98.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mogami N, Nakamura S, Nakamura N. Immunolocalization of the cell wall components in Pinus densiflora pollen. Protoplasma. 1999;206:1–10. [Google Scholar]
- 24.Dhonukshe P, Baluška F, Schlicht M, Hlavacka A, Šamaj J, Friml J, Gadella TWJ., Jr Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev Cell. 2006;10:137–150. doi: 10.1016/j.devcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Otegui MS, Mastronarde DN, Kang B-H, Bednarek SY, Staehelin LA. Three-dimensional analysis of syncytial-type cell plates during endosperm cellularization visualized by high resolution electron tomography. Plant Cell. 2001;13:2033–2051. doi: 10.1105/TPC.010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seguí-Simarro JM, Staehelin LA. Cell cycle-dependent changes in Golgi stacks, vacuoles, clathrin-coated vesicles and multivesicular bodies in meristematic cells of Arabidopsis thaliana: A quantitative and spatial analysis. Planta. 2006;223:223–236. doi: 10.1007/s00425-005-0082-2. [DOI] [PubMed] [Google Scholar]
- 27.Baluška F, Menzel D, Barlow PW. Cytokinesis in plant and animal cells: Endosomes ‘shut the door’. Dev Biol. 2006;294:1–10. doi: 10.1016/j.ydbio.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 28.Evert RF, Eichhorn SE. Sieve-element ultrastructure in Platycerium bifurcatum and some other polypodiaceous ferns: The nacreous wall thickening and maturation of the protoplast. Am J Bot. 1976;63:30–48. [Google Scholar]


