Abstract
External physical and chemical stimuli are transduced via second messengers, following primary interaction with specific membrane or soluble receptors. Ca2+ is an important second messenger in plants as in other eukaryotes, mediating responses to numerous environmental stimuli and affecting a multitude of cellular processes including gene expression. However, there is yet very little information concerning the cis-elements that mediate Ca2+-responsive gene expression. In this article we discuss a recent investigation combining bioinformatics with experimental data, revealing DNA regulatory elements that convey specific cytosolic Ca2+ transients to the transcription machinery.
Key Words: calcium, signal transduction, gene expression, transcription factors, abscisic acid, stress responses
In response to environmental stimuli, including abiotic (cold, heat, salt, drought, light, touch) and biotic stresses, Ca2+ concentrations are transiently elevated, via an increased Ca2+ influx.1 Ca2+ transients are transduced by Ca2+-binding proteins, many of which contain an ‘EF-hand’ motif, which is a helix-loop-helix structure with high specificity for Ca2+ binding.2 Ca2+ transducers include calmodulin (CaM) and CaM-like proteins (CMLs),3,4 Ca2+-dependent protein kinases,5 calcineurin-B-like proteins (CBL),6 potassium7 and Ca2+-channels.8 These affect numerous downstream targets and cellular processes including metabolism,9 cellular structures10 and gene expression.11,12
The apparent complexity of these responses raises questions concerning the mechanisms underlying stimulus-response specificity and cross-talk with other cellular processes.13–15 Studies of Ca2+ signaling in plants and animals suggest that the intracellular Ca2+ signals carry different elements of information including duration of the signals, amplitude, and frequency of oscillations.16–19 These signals need to be decoded17 and translated to the appropriate cellular responses. However, in spite of the obvious importance of Ca2+ signaling, little is known about the mechanisms mediating Ca2+-responsive gene expression in plants. In the discussed paper, Ca2+-responsive genes and cis-elements in Arabidopsis were characterized. The implications and open questions emerging from these studies are discussed.
The complexity of the Ca2+ signaling machinery and stress-induced cellular signaling makes it difficult to distinguish the effects of the Ca2+-response per se from other effects evoked by the same stimuli that induce the Ca2+ signals. For example, ABA activates both Ca2+-dependent and -independent pathways.15, 20 In addition, subcellular compartmentalization of Ca2+ signaling is also a complex issue that needs to be addressed.21 One approach to identify Ca2+-specific responses is by induction of artificial Ca2+ transients with or without Ca2+-signaling inhibitors (e.g., channel blockers, protein inhibitors). Artificial cytosolic Ca2+ transients may be achieved by repeatedly changing the ionic composition of the extra-cellular environment from a depolarizing to hyperpolarizing buffer.16 This type of artificial manipulation revealed the physiological importance of the frequency of Ca2+ oscillations.16 In addition, because several plant Ca2+ transporters are regulated by CaM,4 exposure of plants to CaM antagonists may also alter their Ca2+ homeostasis. Indeed, using aequorin-based luminometry and photon imaging it was shown in the discussed study that CaM antagonists including TFP, W7, calmidazolium and SKF-7171, induced rapid transient changes in cytosolic Ca2+ in intact Arabidopsis and tobacco seedlings. These Ca2+ bursts peaked 30–100 sec after the addition of the CaM antagonists, lasting 5–10 min, with an elevation of at least 10-fold of cytosolic Ca2+ over the measured basal levels. The Ca2+ signals triggered by these antagonists were completely inhibited by the Ca2+-channel blockers, lanthanum and gadolinium, but not by potassium channel blockers.
Analysis of transcriptome changes 1-hr post stimulus revealed 230 Ca2+-responsive genes, of which 162 were upregulated and 68 downregulated. Considering that the chip used in this study represented only 25% of Arabidopsis genome, the total number of upregulated genes might be ∼650, comprising some 2.3% of the whole genome, and a total of 280 downregulated genes, comprising 1% of the genome, for this specific Ca2+ response. The upregulated genes contained more TFs, post-transcriptional regulatory proteins, and signaling-related genes, whereas the downregulated genes included more transporters, specifically aquaporins, and defense-related genes, particularly peroxidases. Thus, although the induced cytosolic Ca2+ burst was artificial, it allowed the identification of genes responding to a cytosolic Ca2+ burst, in the absence of an applied environmental stress. However, many of the upregulated genes were found to be known early stress-responsive genes, such as the touch-responsive genes TCH2 (CML24), TCH3 (CML12) and TCH4-like, the cold-induced genes COR47 and KIN2 and the dehydration early-responsive genes ERD1, ERD6, ERD10, ERD13, ERD14 and ERD15.
Bioinformatic analysis of the 5′ upstream regions of 162 of the upregulated genes revealed a DNA element associated with Ca2+-responsive upregulated gene expression (p < 10−13). The sequence motif [C/A)ACG(T/C)G(T/G/C)] includes the ABRE consensus (ACGTG) and the ABRE-CE core (ACGCG), reported as a functional equivalent of the classical ABREs.22 The analysis also showed that overrepresentation of the ABRE-related motifs occurred exclusively in the upregulated genes. Subsequently, to verify the bioinformatic predictions, a tetramer of the classical ABRE cis-element was tested and found sufficient to confer transcriptional activation in response to cytosolic Ca2+ transients, suggesting that ABREs function as Ca2+-responsive cis-elements at least in some promoter combinations and Ca2+ signaling pathways. However, this does not imply that every ABRE-related promoter sequence is a Ca2+-responsive cis-element, or that every cytosolic Ca2+ signal is transduced by an ABRE-related regulatory element. Ca2+ signals are also associated with the rapid oxidative burst leading to the formation of ROS,23 which plays a role in both biotic and abiotic signaling.24 In this context it is interesting to note that CaM antagonists (e.g., W7), which induced the cytosolic Ca2+ transients in the discussed manuscript, also induced rapid and transient ROS (Fluhr R, personal communication).
Therefore, the results in the discussed paper raise several questions; How Ca2+ signals are transduced to the transcription machinery at the ABRE cis-elements? Are these Ca2+ signals transduced directly to TFs containing EF-hands, or through TFs that respond to a Ca2+-binding protein (e.g., CaM and CMLs), or through other Ca2+-responsive proteins like kinases, or phosphatases? Do these Ca2+ transduction pathways involve components of ABA-signaling, and if not, how do these Ca2+-transduction pathways interact with ABA-signaling pathways operating at the same DNA regulatory elements? How do these Ca2+ signaling pathways interact with ROS-signaling in relation to ABA responses? Are these Ca2+ signals transduced by downstream ROS signals? Figure 1 depicts some of these possibilities and open questions.
Figure 1.
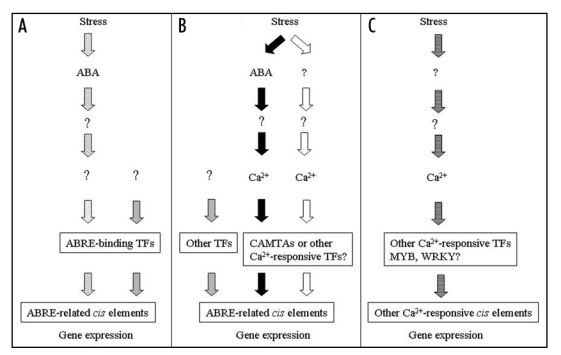
Possible transduction pathways of stress signals to Ca2+-responsive and/or ABA-responsive cis elements. (A) ABA signaling, independent of Ca2+ (dotted arrows); (B) Ca2+ signaling induced by ABA (black arrows), or other stress induced factors, independent of ABA (white arrows), coinciding at ABRE-related cis-elements; (C) Stress-induced Ca2+ signaling acting at cis-elements different from ABREs (stripped arrows); Gray arrows: other signaling pathways not involving ABA or Ca2+; Question marks indicate unknown signaling intermediates.
Independently, a novel family of Ca2+-dependent CaM-binding TFs, designated CAMTAs (or AtSRs) has been characterized in plants12,25,26 and in other multicellular organisms.27,28 In plants, these TFs may function as a link between Ca2+ signaling and ABRE-related cis-elements. The DNA-binding specificity of CAMTAs was shown to match both the ABRE-CE core sequence (ACGCGT/G/C) and the classical ABRE (ACGTGT), though with somewhat lower affinity12 (Finkler A, Fromm H, unpublished results). Interestingly, Choi et al.12 showed that CAMTA-dependent activation of gene expresssion via a synthetic CAMTA-binding site is inhibited by Ca2+/CaM. Putative candidates for activation or repression by CAMTAs via ABRE-related cis-elements are members of the DREB1 family.29 Analysis of DREB1 promoters revealed a number of motifs that correspond to classical ABRE (ACGTG), ABRE-CE motif (CCGCGT or ACGCGG), and ICEr1 motif (CACATG). DREB1A, B and C—all contain in their promoters the ABRE core sequence (ACGTG) with various flanking sequences. The variations in the flanking sequences may impose different affinities for CAMTA binding (Finkler A, Fromm H, unpublished results), with possible implications on fine- tuning of DREB1 expression.
In summary, different approaches may reveal other Ca2+-responsive cis-elements and Ca2+-responsive TFs in plants. In this context, it should be mentioned that a CaM-binding MYB transcription factor was found to enhance salt tolerance in Arabidopsis,11 and Arabidopsis WRKY group IId TFs also bind CaM.30 Thus, certain Ca2+-transduction pathways in plants operate through CaM-binding TFs to modulate gene expression. We found that the Ca2+/CaM-responsive CAMTAs bind to the same Ca2+-responsive cis-elements that were identified in the discussed study, suggesting a link between Ca2+-responsive TFs and ABRE-related cis-elements. This link merits further investigations in the context of ABA, ROS signaling and stress responses.
Abbreviations
- CaM
calmodulin
- TF
transcription factor
- ABA
abscisic acid
- ABRE
ABA-responsive element
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3611
References
- 1.Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plants aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- 2.Day IS, Reddy VS, Shad Ali G, Reddy AS. Analysis of EF-hand-containing proteins in Arabidopsis. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-10-research0056. RESEARCH0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacCormack E, Braam J. Calmodulins and related potential calcium sensors in Arabidopsis. New Phytol. 2003;159:585–598. doi: 10.1046/j.1469-8137.2003.00845.x. [DOI] [PubMed] [Google Scholar]
- 4.Bouché N, Yellin A, Snedden WA, Fromm H. Plant-specific calmodulin-binding proteins. Annu Rev Plant Biol. 2005;56:435–466. doi: 10.1146/annurev.arplant.56.032604.144224. [DOI] [PubMed] [Google Scholar]
- 5.Harper JF, Breton G, Harmon A. Decoding Ca2+ signals through plant protein kinases. Annu Rev Plant Biol. 2004;55:263–288. doi: 10.1146/annurev.arplant.55.031903.141627. [DOI] [PubMed] [Google Scholar]
- 6.Luan S, Kudla J, Rodrigues-Concecion M, Yalovsky S, Gruissem W. Calmodulins and calcineurin B-like proteins: Calcium sensors for specific signal response coupling in plants. Plant Cell. 2002;14:S389–S400. doi: 10.1105/tpc.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czempinski K, Zimmermann S, Ehrhardt T, Muller-Rober B. New structure and function in plant K+ channels: KCO1, an outward rectifier with a steep Ca2+ dependency. EMBO J. 1997;16:2565–2575. doi: 10.1093/emboj/16.10.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 9.Snedden WA, Koutsia N, Baum G, Fromm H. Activation of a recombinant petunia glutamate decarboxylase by calcium/calmodulin or by a monoclonal antibody which recognizes the calmodulin binding domain. J Biol Chem. 1996;271:4148–4153. doi: 10.1074/jbc.271.8.4148. [DOI] [PubMed] [Google Scholar]
- 10.Malhó R, Trewavas AJ. Localized apical increases of cytosolic free calcium control pollen tube organization. Plant Cell. 1996;8:1935–1949. doi: 10.1105/tpc.8.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo JH, Park CY, Kim JC, Heo WD, Cheong MS, Park HC, Kim MC, Moon BC, Choi MS, Kang YH, Lee JH, Kim HS, Lee SM, Yoon HW, Lim CO, Yun DJ, Lee SY, Chung WS, Cho MJ. Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J Biol Chem. 2005;280:3697–3706. doi: 10.1074/jbc.M408237200. [DOI] [PubMed] [Google Scholar]
- 12.Choi MS, Kim MC, Yoo JH, Moon BC, Koo SC, Park BO, Lee JH, Koo YD, Han HJ, Lee SY, Chung WS, Lim CO, Cho MJ. Isolation of a calmodulin-binding transcription factor from Rice (Oryza sativa L.) J Biol Chem. 2005;280:40820–40831. doi: 10.1074/jbc.M504616200. [DOI] [PubMed] [Google Scholar]
- 13.Trewavas AJ, Malho R. Ca2+ signalling in plant cells: The big network! Curr Opin Plant Biol. 1998;1:428–433. doi: 10.1016/s1369-5266(98)80268-9. [DOI] [PubMed] [Google Scholar]
- 14.Bowler C, Fluhr R. The role of calcium and activated oxygens as signals for controlling cross-toleranc. Trends Plant Sci. 2000;5:241–246. doi: 10.1016/s1360-1385(00)01628-9. [DOI] [PubMed] [Google Scholar]
- 15.Knight H, Knight MR. Abiotic stress signalling pathways: Specificity and cross-talk. Trends Plant Sci. 2001;6:262–267. doi: 10.1016/s1360-1385(01)01946-x. [DOI] [PubMed] [Google Scholar]
- 16.Allen GJ, Chu SP, Schumacher K, Shimizaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, Chory J, Schroeder JI. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science. 2000;289:2338–2342. doi: 10.1126/science.289.5488.2338. [DOI] [PubMed] [Google Scholar]
- 17.Allen JP, Schroeder JI. Combining genetics and cell biology to crack the code of plant cell calcium signaling. Sciences STKE. 2001;102:RE13. doi: 10.1126/stke.2001.102.re13. [DOI] [PubMed] [Google Scholar]
- 18.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hetherington AM, Brownlee C. The generation of Ca2+ signals in plants. Annu Rev Plant Biol. 2004;55:401–427. doi: 10.1146/annurev.arplant.55.031903.141624. [DOI] [PubMed] [Google Scholar]
- 20.Allan AC, Fricker MD, Ward JL, Beale MH, Trewavas AJ. Two transduction pathways mediate rapid effects of abscisic acid in commelina guard cells. Plant Cell. 1994;6:1319–1328. doi: 10.1105/tpc.6.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong TC, Bourque S, Lecourieux D, Amelot N, Grat S, Briere C, Mazars C, Pugin A, Ranjeva R. Calcium signaling in plant cell organelles delimited by a double membrane. Biochem Biophys Acta. 2006;1763:1209–1215. doi: 10.1016/j.bbamcr.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Hobo T, Asada M, Kowyama Y, Hattori T. ACGT-containing abscisic acid response element (ABRE) and coupling element 3 (CE3) are functionally equivalent. Plant J. 1999;19:679–689. doi: 10.1046/j.1365-313x.1999.00565.x. [DOI] [PubMed] [Google Scholar]
- 23.Sagi M, Fluhr R. Superoxide production by plant homologues of the gp91phox NADPH oxidase: Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol. 2001;126:1281–1290. doi: 10.1104/pp.126.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Cross-talk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Bouché N, Scharlat A, Snedden W, Bouchez D, Fromm H. A novel family of Calmodulin-binding transcription activators in multicellular organisms. J Biol Chem. 2002;277:50597–50606. doi: 10.1074/jbc.M200268200. [DOI] [PubMed] [Google Scholar]
- 26.Yang T, Poovaiah BW. A calmodulin-binding, CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J Biol Chem. 2002;277:45049–45058. doi: 10.1074/jbc.M207941200. [DOI] [PubMed] [Google Scholar]
- 27.Han J, Gong P, Reddig K, Mitra M, Guo P, Li HS. The fly CAMTA transcription factor potentiates deactivation of rhodopsin, a G protein-coupled light receptor. Cell. 2006;127:847–858. doi: 10.1016/j.cell.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Song K, Backs J, McAnally J, Qi X, Gerard RD, Richardson JA, Hill JA, Bassel-Duby R, Olson EN. The transcriptional coactivator CAMTA2 stimulates cardiac growth by opposing class II histone deacetylases. Cell. 2006;125:453–466. doi: 10.1016/j.cell.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 29.Taleb F, Fromm H. Calcium/calmodulin-binding transcription activators in plants and animals. In: Evans DE, Bryant L, editors. The Nuclear Envelope. UK: Bioscientific Publishers Ltd.; 2004. [Google Scholar]
- 30.Park CY, Lee JH, Yoo JH, Moon BC, Choi MS, Kang YH, Lee SM, Kim HS, Kang KY, Chung WS, Lim CO, Cho MJ. WRKY group IId transcription factors interact with calmodulin. FEBS Lett. 2005;579:1545–1550. doi: 10.1016/j.febslet.2005.01.057. [DOI] [PubMed] [Google Scholar]


