Abstract
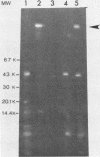
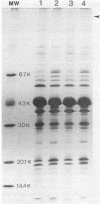
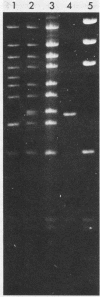
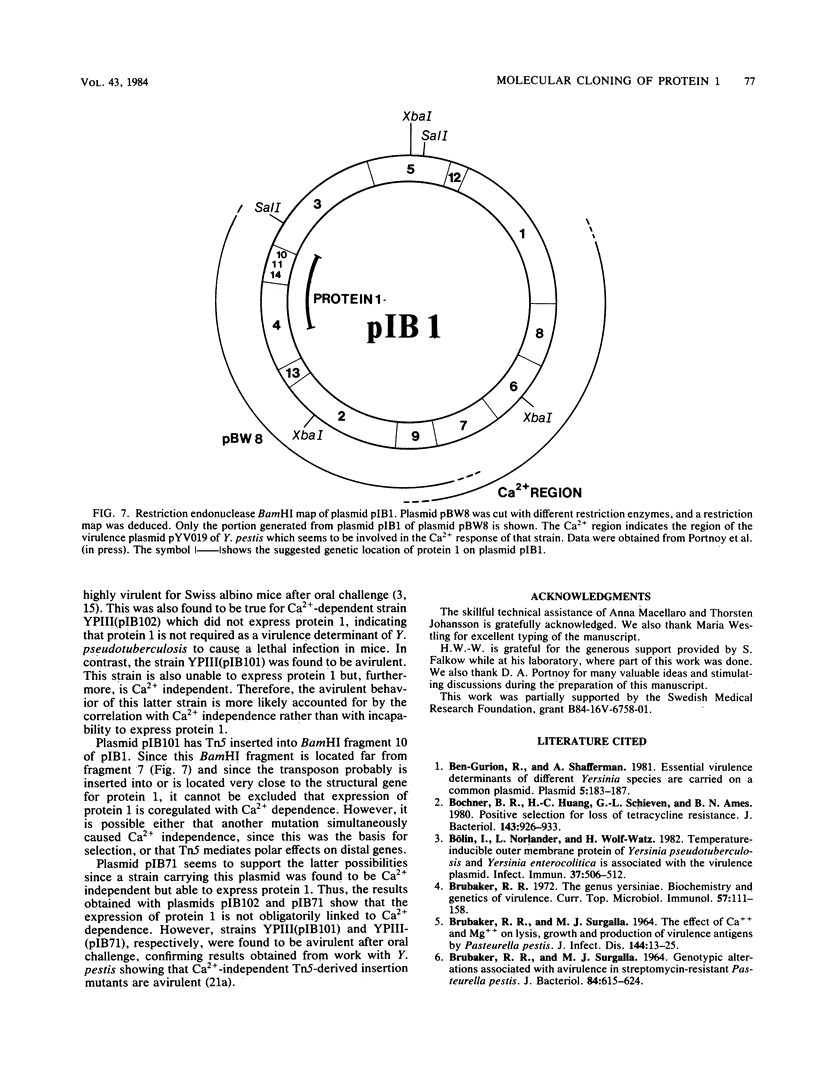
When Yersinia pseudotuberculosis is shifted from growth at 26 degrees C to growth at 37 degrees C, the synthesis of a plasmid-associated outer membrane protein, protein 1, is induced (Bölin et al., Infect. Immun. 37:506-512). The structural gene of this protein was found to be located on the virulence plasmid pIB1 of Y. pseudotuberculosis. One cosmid hybrid plasmid pBW8 was studied which carried a region of the virulence plasmid. This hybrid plasmid expressed in Escherichia coli K-12 a novel temperature-inducible outer membrane protein which is immunologically related to and has the same molecular weight as protein 1. Protein 1 was purified to homogeneity, and 14 amino acids of the N-terminal end were determined. From this sequence, the tentative corresponding DNA sequence was deduced, and a set of 11-nucleotide-long DNA probes was chemically synthesized. By using these probes in Southern blotting experiments, the genetic location of the N-terminal end of protein 1 was established. By introducing the transposon Tn5 into the virulence plasmid pIB1, mutants were obtained that did not express protein 1. One class of these mutants was still Ca2+ dependent and virulent, suggesting that protein 1 is not a major virulence determinant. Tn5-derived insertion mutants were also obtained which were Ca2+ independent. Such mutants were found to be avirulent. One Ca2+-independent mutant still expressed protein 1, indicating that the regulatory expression of protein 1 is not linked to Ca2+ dependence.
Full text
PDF


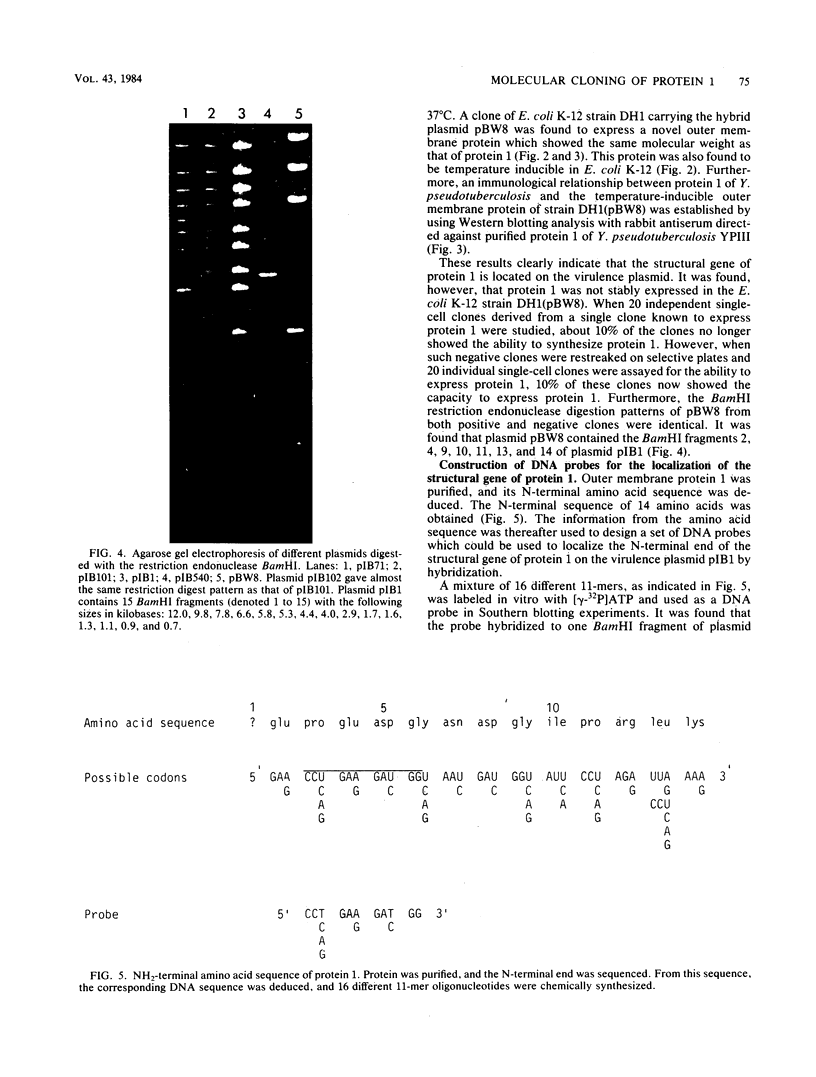


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON G. A., BURROWS T. W. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956 Oct;37(5):481–493. [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. Genotypic alterations associated with avirulence in streptomycin-resistant Pasteurella pestis. J Bacteriol. 1962 Oct;84:615–624. doi: 10.1128/jb.84.4.615-624.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. THE EFFECT OF CA++ AND MG++ ON LYSIS, GROWTH, AND PRODUCTION OF VIRULENCE ANTIGENS BY PASTEURELLA PESTIS. J Infect Dis. 1964 Feb;114:13–25. doi: 10.1093/infdis/114.1.13. [DOI] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. V and W antigens in strains of Pasteurella pseudotuberculosis. Br J Exp Pathol. 1960 Feb;41:38–44. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W. VIRULENCE OF PASTEURELLA PESTIS AND IMMUNITY TO PLAGUE. Ergeb Mikrobiol Immunitatsforsch Exp Ther. 1963;37:59–113. doi: 10.1007/978-3-662-36742-1_2. [DOI] [PubMed] [Google Scholar]
- Ben-Gurion R., Shafferman A. Essential virulence determinants of different Yersinia species are carried on a common plasmid. Plasmid. 1981 Mar;5(2):183–187. doi: 10.1016/0147-619x(81)90019-6. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol Immunol. 1972;57:111–158. doi: 10.1007/978-3-642-65297-4_4. [DOI] [PubMed] [Google Scholar]
- Bölin I., Norlander L., Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982 Aug;37(2):506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Zahorchak R. J., Brubaker R. R. Plague virulence antigens from Yersinia enterocolitica. Infect Immun. 1980 May;28(2):638–640. doi: 10.1128/iai.28.2.638-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmblad A., Josephson S., Palm G. Synthesis of mixed oligodeoxyribonucleotides following the solid phase method. Nucleic Acids Res. 1982 May 25;10(10):3291–3301. doi: 10.1093/nar/10.10.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson U., Sundelin J., Rask L., Peterson P. A. The NH2-terminal amino acid sequence of cellular retinoic-acid binding protein from rat testis. FEBS Lett. 1981 Nov 30;135(1):70–72. doi: 10.1016/0014-5793(81)80945-3. [DOI] [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. Plasmids in Yersinia pestis. Infect Immun. 1981 Feb;31(2):839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T., Wohlhieter J. A. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun. 1980 Jun;28(3):1044–1047. doi: 10.1128/iai.28.3.1044-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961 Apr;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- LAWTON W. D., ERDMAN R. L., SURGALLA M. J. BIOSYNTHESIS AND PURIFICATION OF V AND W ANTIGEN IN PASTEURELLA PESTIS. J Immunol. 1963 Aug;91:179–184. doi: 10.21236/ad0299868. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Vwa+ phenotype of Yersinia enterocolitica. Infect Immun. 1983 Apr;40(1):166–171. doi: 10.1128/iai.40.1.166-171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Blank H. F., Kingsbury D. T., Falkow S. Genetic analysis of essential plasmid determinants of pathogenicity in Yersinia pestis. J Infect Dis. 1983 Aug;148(2):297–304. doi: 10.1093/infdis/148.2.297. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Falkow S. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J Bacteriol. 1981 Dec;148(3):877–883. doi: 10.1128/jb.148.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Mayer L. W., Tam M. R. Antigenicity of Neisseria gonorrhoeae outer membrane protein(s) III detected by immunoprecipitation and Western blot transfer with a monoclonal antibody. Infect Immun. 1982 Nov;38(2):668–672. doi: 10.1128/iai.38.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trägårdh L., Curman B., Wiman K., Rask L., Peterson P. A. Chemical, physical-chemical, and immunological properties of papain-solubilized human transplatation antigens. Biochemistry. 1979 May 29;18(11):2218–2226. doi: 10.1021/bi00578a013. [DOI] [PubMed] [Google Scholar]
- Zink D. L., Feeley J. C., Wells J. G., Vanderzant C., Vickery J. C., Roof W. D., O'Donovan G. A. Plasmid-mediated tissue invasiveness in Yersinia enterocolitica. Nature. 1980 Jan 10;283(5743):224–226. doi: 10.1038/283224a0. [DOI] [PubMed] [Google Scholar]