Abstract
Calcium ion (Ca2+) is one of the very important ubiquitous intracellular second messenger molecules involved in many signal transduction pathways in plants. The cytosolic free Ca2+ concentration ([Ca2+]cyt) have been found to increased in response to many physiological stimuli such as light, touch, pathogenic elicitor, plant hormones and abiotic stresses including high salinity, cold and drought. This Ca2+ spikes normally result from two opposing reactions, Ca2+ influx through channels or Ca2+ efflux through pumps. The removal of Ca2+ from the cytosol against its electrochemical gradient to either the apoplast or to intracellular organelles requires energized ‘active’ transport. Ca2+-ATPases and H+/Ca2+ antiporters are the key proteins catalyzing this movement. The increased level of Ca2+ is recognised by some Ca2+-sensors or calcium-binding proteins, which can activate many calcium dependent protein kinases. These kinases regulate the function of many genes including stress responsive genes, resulted in the phenotypic response of stress tolerance. Calcium signaling is also involved in the regulation of cell cycle progression in response to abiotic stress. The regulation of gene expression by cellular calcium is also crucial for plant defense against various stresses. However, the number of genes known to respond to specific transient calcium signals is limited. This review article describes several aspects of calcium signaling such as Ca2+ requiremant and its role in plants, Ca2+ transporters, Ca2+-ATPases, H+/ Ca2+-antiporter, Ca2+-signature, Ca2+-memory and various Ca2+-binding proteins (with and without EF hand).
Key Words: Calcium binding proteins, Ca2+ channel, Ca2+-dependent protein kinases, Ca2+/H+ antiport, calcium memory, calcium sensors, calcium signatures, Ca2+-transporters, EF hand motifs, plant signal transduction
Introduction
Calcium plays a fundamental role in plant's growth and development. Many extracellular signals and environmental cues including light, abiotic and biotic stress factors, elicit change in the cellular calcium levels, termed as calcium signatures.1 Ca2+ has been well established as a second messenger and the concentration of Ca2+ is delicately balanced by the presence of ‘Ca2+ stores’ like vacuoles, endoplasmic reticulum, mitochondria and cell wall. Ca2+ is present in milimolar concentrations in the cell wall and vacuoles and is released whenever required by the cell.1, 2 Recently, Xiong et al. (2006)3 have demonstrated that the organelles surrounded by a double membrane (e.g. mitochondria, chloroplasts and nuclei) are also equipped to generate calcium signal on their own, which is delimited by a double membrane.
Ca2+ ion represents an important signaling molecule and a convergence point of many disparate signaling pathways. Facing the environmental challenge, plant cells reprogram their cellular set up by triggering a network of signaling events that start with stress perception at the membrane level of the cell and ends with a cellular response (Fig. 1). A generic signal transduction pathway has following steps:
Figure 1.
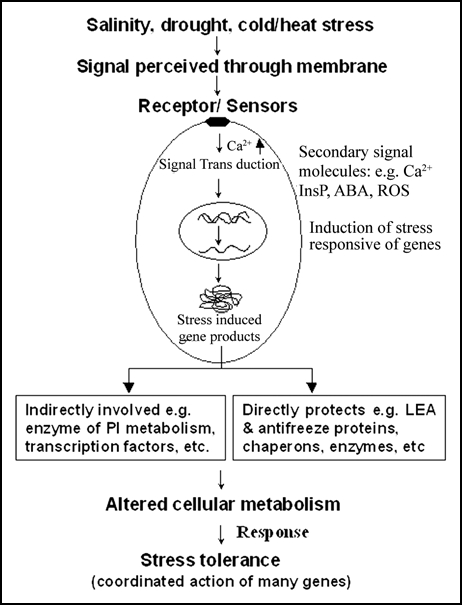
Generic pathway for plant response to stress. The extracellular stress signal is first perceived by the membrane receptors and then activate large and complex signaling cascade intracellularly including the generation of secondary signal molecules. The signal cascade results into the expression of multiple stress responsive genes, the products of which can provide the stress tolerance directly or indirectly. ABA, abscisic acid; LEA, late embryogenesis abundant; InsP, inositol phosphate; ROS, reactive oxygen species.
(a) Perception of the signal by the membrane receptors; (b) Generation of second messengers; (c) A cascade of protein phosphorylation/dephosphorylation events that may target transcription factors controlling specific set of stress regulated genes; (d) Stress tolerance, plant adaptation and other phenotypic responses.
The extracellular stress signal transduces inside the nucleus to induce multiple stress responsive genes, the products of which ultimately lead to plant adaptation to stress tolerance directly or indirectly (Fig. 1).1 Overall, the stress response could be a coordinted action of many genes, which may cross-talk with each others. Signal transduction requires the proper spatial and temporal coordination of all signaling molecules. In plant cell, many molecules acting as second messenger in signaling pathways have been reported; these include Ca2+, lipids like IP3 and cyclic GMP (cGMP). However no single messenger except cytosolic Ca2+ has been demonstrated to be involved in diverse pathways and respond to numerous stimuli. A generic pathway for calcium regulated expression of stress responsive genes is shown in Figure 2. The elevated level of Ca2+ can be recognized by calcium sensors or Ca2+-binding proteins, which together can activate protein kinases.1, 2 These activated protein kinases can phosphorylate many regulatory proteins including transcription factors, which regulate the gene expression level of regulatory proteins resulting in the altered metabolism followed by phenotypic response of stress tolerance (Fig. 2). The response could also be growth inhibition or cell death, which will depend upon how many and what kinds of genes are getting upregulated or down-regulated in response to the elevated calcium.
Figure 2.
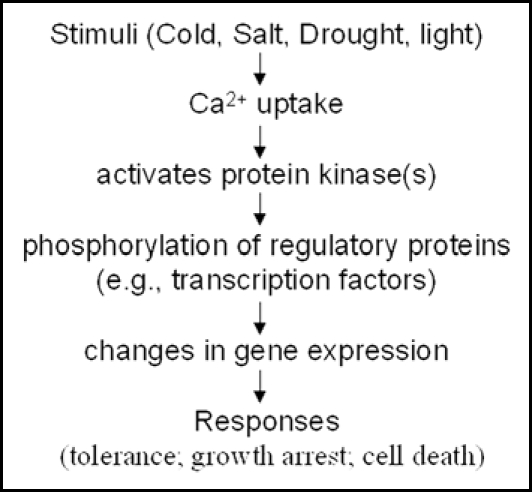
A generic pathway for calcium regulated gene expression and the stress response. The calcium level increases in response to the stimuli. The increased level of Ca2+ is recognised by some Ca2+-sensors or calcium-binding proteins, which can activate many calcium dependent protein kinases. These kinases regulate the function of many genes including stress responsive genes, resulted in the phenotypic response of stress tolerance.
Overall, in response to calcium several genes are reported to be upregulated. An analysis of transcriptome changes revealed 230 calcium-responsive genes, of which 162 were upregulated and 68 were downregulated. These include known early stress-responsive genes as well as genes of unknown function.4 Recently, a blue light receptor phototropins, which regulate growth and development of plants, has also been shown to involved in calcium signaling in higher plants.5 Ca2+ signaling pathway can also regulates a K+ channel for low-K response in Arabidopsis. Calcium is also reported to be an essential component of the sucrose signaling pathway that leads to the induction of fructan synthesis.6 Calcium signaling is also involved in the regulation of cell cycle progression in response to abiotic stress. Understanding the immense significance of Ca2+ ions, this review has been solely dedicated to the salient features associated with calcium signaling. Various aspects regarding the Ca2+ requirements of plant, Ca2+ deficiency, Ca2+ transporters, efflux pumps, Ca2+/H+ antiporters, Ca2+ signatures, Ca2+ memory, Ca2+ sensor and transducer proteins have been briefly covered in this review.
Role of calcium in plants
Calcium is an essential plant nutrient required for growth and development of plant, especially the root and shoot tip. The tips are meristmatic and cell division occurs by mitosis. Ca2+ helps in the formation of microtubules and microtubules in turn are essential for the anaphasic movement of chromosomes.
Ca2+ is an important divalent cation and is required for structural roles in the cell wall and membranes where it exists as Ca2+ pectate. Ca2+ accumulates as calcium pectate in the cell wall and binds the cells together.
It is also required as a counter-cation for inorganic and organic anions in the vacuole and as an intracellular messenger in the cytosol.1 Externally supplied Ca2+ reduces the toxic effects of NaCl and ameliorates stress.
Ca2+ is required for pollen tube growth and elongation.8
The calcium requirement of plants and its uptake from soil
Calcium was first described as an essential macro nutrient element in plants more than a century ago.9 Ca2+ is taken up by roots from the soil solution and delivered to the shoot via the xylem. Ca2+ may traverse the root either through the cytoplasm of the cells, linked together by plasmodesmata (the symplast) or through the spaces between the cells (the apoplast). The cytosolic free Ca2+ concentration is maintained typically at 200 nM.10 However, the Ca2+ content of the cytosol is far higher than this because of the high affinity of Ca2+ to a range of Ca2+ binding proteins. Ca2+ mobility within the cell is very low as a consequence of its low free concentration and rapid chelation. It moves predominantly apoplastically rather than symplastically.
Plants vary markedly in their Ca2+ content and requirements. Ca2+ content can very between 0.1 and >5.0% dry wt.11 for different plants and organs. Soil Ca2+ also varies widely from <0.01% calcium in acid laterites to very high abundance in chalky soils.9 Ecologists have classified plant species into calcifuges and calcicoles. Calcifuges are plants occurring on acidic soils with low Ca2+. The term calciole is used to describe plants, which can grow on calcareous/Ca2+ rich soils. These soils are generally Ca2+ and base-rich soils. However, plants vary in their Ca2+ requirements and the ability to extract Ca2+ from complex soil environments. In particular, monocots require less Ca2+ for optimal growth than do the dicots.12 Ca2+ enters plant cells through Ca2+-permeable ion channels in their plasma membranes. Ca2+ competes with other cations both for these sites and for the uptake from the soil. The presence of high levels of Ca2+ is known to ameliorate the effects of the uptake of toxic cations (Al3+ and Na+) from the soil while the presence of high levels of other cations (K+, Mg2+) may reduce Ca2+ uptake. Calcium uptake and requirements for optimal growth are thus strongly dependent on the presence of other cations.9
Calcium deficiency is rare in nature, but may occur on soils with low base saturation and/or high levels of acidic deposition. Calcium deficiency may occur because of competition by other cations, or because of low transpiration, where the xylem flow is inadequate to supply the calcium requirements of rapidly growing tissue. Calcium deficiency results in stunted root growth and altered leaf appearances.11 More severe symptoms generally result from the failure of cell membrane integrity and include bitter-pit in apple fruit, blossom end rot in tomato and tip burn in lettuce. Deficiency symptoms develop first in the regions of plant most distal to the region of Ca2+ uptake.
Ca2+ Transporters/ Efflux Pumps in the Cellular Membranes
Plant cells, like animal cells, maintain a low regulated free Ca2+concentration ranging from 30 nM to 400 nM in higher plants. Maintaining the low cytosolic Ca2+ concentration observed in plant cells requires active transport of Ca2+ from the cytosol. Active efflux pumping is a prerequisite for the restoration of low levels cytosolic calcium after the signaling event. The removal of Ca2+ from the cytosol against its electrochemical gradient to either the apoplast or to intracellular organelles requires energized ‘active’ transport. Ca2+-ATPases and H+/Ca2+ antiporters are the key proteins catalyzing this movement.
By removing Ca2+ from the cytosol several important functions are performed by these enzymes.13
They maintain a low [Ca2+]cyt in the resting or the unstimulated cell which is appropriate for the cytoplasmic metabolism.
They restore [Ca2+]cyt levels to the resting normal levels following a [Ca2+]cyt perturbation, thereby influencing the magnitude kinetic and subcellular location of [Ca2+]cyt signals.
They replenish intracellular and extracellular Ca2+ stores for subsequent [Ca2+]cyt signals and permit the generation of localized [Ca2+]cyt oscillations through their interplay with Ca2+ channels.14
They provide Ca2+ in the ER for the secretory system to function.
They remove some divalent cations, such as Ni2+, Zn2+, Mg2+ and Mn2+ from the cytosol to prevent mineral toxicity.13
Hirschi (2001)13 suggested that the Ca2+-ATPases, which have high affinity (Km = 1–10 µM) but low capacity for Ca2+ transport, are responsible for maintaining [Ca2+]cyt homeostasis in the resting cells. Whereas the H+/Ca2+-antiporters, which have lower affinities (Km = 10–15 µM) but high capacities for Ca2+ transport, are likely to remove Ca2+ from the cytosol during [Ca2+]cyt signals and thereby modulate [Ca2+]cyt perturbations. This hypothesis is supported by the fact that H+/Ca2+ antiporter, but not the vacuolar Ca2+-ATPase, resets [Ca2+]cyt in yeast following hypertonic shock.15
Plant Ca2+-ATPases belongs to two major families (a) The P-type ATPase II A family and (b) The P-type ATPase II B family.16
(a) The P-type ATPase II A family.
Nucleotide specificity of these pumps is broad (30–60% activity achieved with GTP and ITP). The pumps are inhibited by erythrosine B (IC50 ≤ 1 µM) and estimate Ca2+affinity is in the range of 0.4–12 µM. The first family (The P-type ATPase II A family) lacks N-terminal auto regulatory domain. Four members of this family have been identified in the Arabidopsis genome (termed AtECAs 1 to 4 by Axelsen and Palmgren, (2001).16 They are likely to be present in the plasma membrane, tonoplast ER and the Golgi apparatus.
(b) The P-type ATPase II B family.
The second family of plant Ca2+-ATPases (the P-type ATPase IIB family) is characterized by an auto inhibitory N-terminal domain that contains a binding site for Ca-CaM and in addition a serine-residue phosphorylation site. Their catalytic activity can be modulated by [Ca2+]cyt either through activation upon binding CaM or by inhibition following phosphorylation by Ca2+-dependent protein kinases (CDPK).17 Since CaM binding sites are generally quite diverse, each type-II B Ca2+-ATPase may have different affinity for CaM or may bind a different CaM isoform. Ten members of the type-II B, Ca2+-ATPase family have been identified in the Arabidopsis genome (termed at ACAS1, 2 and 4 and ALACAs 7 to 13 by Axelsen and Palmgren, (2001).16 These Ca2+-ATPases reside on various cellular membranes including the plasma membrane (AtACA8), the tonoplast (AtACA4), and the plastid inner membrane (AtACA1). The relative molecular mass of type II B Ca2+-ATPase pumps has been estimated to be between 115,000 Da and 135,000 Da.18
The abundance of Ca2+-ATPase isoforms suggest that individual isoforms are functionally distinct and may respond differentially to distinct cellular processes involving specific Ca2+ signals. They also imply a requirement for CaM-independent and CaM-dependent regulation of Ca2+-ATPase activities in the modulation of [Ca2t]cyt perturbations during cell signaling. The expression of many Ca2+-ATPases is increased upon exposure to high salinity or high [Ca2+]cyt and some Ca2+-ATPase genes are expressed only under stress conditions.19 This may reflect a role in maintaining [Ca2+]cyt homeostasis or in reducing Na+ influx to the cytosol in saline environments.
Ca2+/H+ Antiporters
In addition to a P-type pump, the presence of a low affinity Ca2+/H+ antiport mechanism at the plasma membrane level had been suggested. The first plant H+/Ca2+ antiporter to be cloned and functionally expressed was CAX1 (Calcium exchanger 1).20 The gene was identified by its ability to restore growth on a high Ca2+ medium to a yeast mutant defective in vacuolar Ca2+ transport.
The H+/Ca2+-antiporters present in the plasma membrane and tonoplast have been characterized biochemically.8 These have a lower affinity for Ca2+ than Ca2+-ATPases and may also transport Mg2+. The stoichiometry of the dominant H+/Ca2+-antiporter in the tonoplast is apparently 3H+/1Ca2+. Eleven genes encoding putative H+/Ca2+ antiporters (AtCAX) have been identified in the genome of Arabidopsis thaliana.13 The transporters AtCAX1, AtCAX2 and AtCAX4 are located at the tonoplast.13 The AtCAX1 antiporter exhibits both a high affinity and high specificity for Ca2+. By contrast, the AtCAX2 transporter is a high-affinity, high capacity H+/heavy metal cation antiporter. The AtCAXs have homologues in other plant species and their physiological roles have been investigated using transgenic plants.13 Transgenic tobacco overexpressing AtCAX1 exhibits Ca2+-deficiency disorders, which includes tip burn, metal-hypersensitivity and susceptibility to chilling that can be reversed by increasing Ca2+supply. By raising Ca2+ supply the expression of AtCAX1 and AtCAX3 (but not AtCAX2 or AtCAX4) genes was increased.13
Ca2+/H+ antiporters, utilize the H+ gradient generated by the tonoplast V-type H+-pump and by a proton-pumping pyrophosphatase to sequester Ca2+ in the Vacuole.21 In many plant cells the vacuole occupy more than 50% of the cell volume, and it is evident that trans tonoplast Ca2+ transport makes a very significant contribution to the regulation of cytosolic Ca2+ concentrations. Ca2+ concentrations within the vacuole range from 0.1 to 10 mM.22
Ca2+-ATPases are estimated to represent only <0.1% of the membrane protein and are thus 30–100 fold less abundant than H+-ATPases in the PM (3%) and the endomembranes (5–10%). All Ca2+ pumps are inhibited by orthovanadate. H+/Ca2+ antiporters are efflux transporter and are different from Ca2+-ATPases in that they do not require ATP and they are not sensitive to vanadate.
In contrast Ca2+ influx to the cytosol is mediated via Ca2+ channels. The principal roles of Ca2+ permeable channels in the plasma membrane appear to be in all signaling. Ca2+ permeable channels have been found in all plant membranes. They have been classified on the basis of their voltage-dependence into depolarization activated (DACC), hyperpolarization-activated (HACC) and voltage independent cation channels (VICC).8
The Ca2+ Signatures
The cytosolic Ca2+ in plant cells increases in response to various environmental challenges like abiotic and biotic stresses and developmental cues. This transient increase in [Ca2+]cyt is considered critical for the production of a physiological response. Elevation of [Ca2+]cyt is considered a universal response to stress. The perturbations in cytosolic calcium levels (termed as [Ca2+]cyt “signature”) elicited by each environmental challenge and developmental cue is unique and results in an appropriate physiological response to a particular stimulus. The uniqueness is manifested in the sub-cellular location and/or the kinetics of magnitude of the [Ca2+]cyt perturbation.23 An increase in [Ca2+]cyt is effected by Ca2+ influx to the cytosol either from the apoplast, across the plasma membrane, or from the intracellular organelles. The Ca2+ influx is mediated by Ca2+ permeable ion channels, and their type, cellular localization and the abundance influences the spatial characteristics of [Ca2+]cyt perturbations. Since the diffusion of Ca2+ within the cytoplasm is low, and the buffering of Ca2+ in the cytoplasm is high (0.1 to 1 mM)24 the opening of Ca2+ channel produces a local increase in [Ca2+]cyt that dissipates rapidly after the channel has been closed. The subcellular localization of Ca2+ channel is therefore critical for the targeting of different cellular processes. Cytosolic calcium “waves” are produced within the cytoplasm by the successive recruitment of particular Ca2+ channels to coordinate cellular responses. It was suggested that a local elevation of [Ca2+]cyt might generate soluble second messengers, such as IP3 or cADPR, that diffuse through the cytoplasm to activate a relay of spatially separated Ca2+ channels.24 This theory was supported in the plant cells responding to salt stress.25
Franklin-Tong et al. (2002)26 suggested that repetitive Ca2+ influx across the plasma membrane contributed in the [Ca2+]cyt that occur following the application of ABA to guard cells. This transient increase was first close to the plasma membrane and subsequently adjacent to the vacuole.27 These waves are thought to reflect the sequential opening of hyperpolarization-activated Ca2+ channels at the plasma membrane and then second-messenger activated Ca2+ channels in the tonoplast.28
In addition to these sub cellular waves, “waves” of cells with high [Ca2+]cyt may also propagate through the plant tissue. This can be induced in root tissues by mechanical stimulation29 or saline shock30, in cotyledons by cold shock31 and in leaves by chilling plant roots briefly.32 Electrical action potentials, osmotic perturbations or chemical signals may trigger these waves. Although an elevated [Ca2+]cyt is necessary for signal transduction, a prolonged increase in [Ca2+]cyt is lethal. Sustained high [Ca2+]cyt is implicated in apoptosis, both during the normal development and in hypersensitive responses to pathogens.33
To effect adaptive responses, the [Ca2+]cyt perturbations must be either of low amplitude or transient. Transient increases in [Ca2+]cyt can be single (spike), double (biphasic) or multiple (oscillations). The perturbations generated may differ in their cellular location, role and extent of propagation and on their amplitude during propagation. Calcium signatures may also be tissue specific. For example, within the root, the [Ca2+ ]cyt perturbations induced by mechanical perturbation, salinity, osmotic stress, cold shock or slow cooling differ markedly between cell types.30
Several abiotic challenges result in an immediate, transient increase in [Ca2+]cyt that is restored to basal levels within minutes. Such changes include mechanical perturbations and rapid cooling for brief periods, termed as ‘cold shock’. The duration, periodicity and amplitude of oscillations vary considerably, and their form is often dependent on the strength and combination of specific stimuli.27
Calcium “Memory”
The term “memory” was put forward first by Knight et al. (1996).34 There is considerable evidence that [Ca2+]cyt signatures are modified by previous experience. A diminished [Ca2+]cyt elevation upon repetitive stimulation by the same environmental challenge or a developmental cue is a common observation. Many examples support this. The magnitude of the [Ca2+]cyt perturbation elicited by the wind-induced motion becomes progressively smaller upon repeated stimulation and a refractory period of several minutes is required before a full response is observed again. A second exposure to an elicitor does not influence [Ca2+]cyt for several hours after its initial application.35 For example, plant cells challenged with H2O2 fail to respond to H2O2 for several hours.36 There is also evidence that the [Ca2+]cyt signatures elicited by one environmental challenge can be modified by prior exposure to a contrasting one. For example, the magnitude of the [Ca2+]cyt perturbations in response to oxidative stress was reduced by prior exposure to hyperosmotic stress and vice-versa was also found to be true. These observations also imply a cross talk between the signaling cascades. The attenuated response of [Ca2+]cyt after repeated stimulation by various elicitors forms a part of cellular memory and the cells are able to retain the previous information. This ’memory’ is significant and helps the cells to respond better to a particular stress without disturbing the delicate balance of Ca2+ levels and maintaining cellular [Ca2+] homeostasis. It is noteworthy that Nicotiana plumbaginifolia, which could not retain ‘cold memory’ during acclimation of the plant by pretreatment with nonfreezing cold temperature was cold-sensitive whereas Arabidopsis which could retain ‘cold memory’ was more resistant to cold stress.34
Calcium Binding Proteins
Transient increase in the cytoplasmic Ca2+ in response to signals is sensed by an array of Ca2+ sensors. Ca2+ sensors are small proteins that bind Ca2+ and change their conformation in a Ca2+ dependent manner. Specificity in the signaling pathway is provided by the uniqueness in calcium signatures and also by plethora of Ca2+ sensors, which can decode the Ca2+ perturbations quite precisely. Once Ca2+ sensors decode the elevated [Ca2+]cyt, Ca2+efflux into the cell exterior and/or the sequestration into cellular organelles such as vacuoles, ER and mitochondria restores its levels to resting state. The properties of binding proteins are described below:
Properties essential for Ca2+-binding proteins (CaBPs) to function as intracellular-Ca2+receptor.
The Ca2+ receptor/sensor must have Ca2+binding sites essentially unoccupied in the resting cell (free Ca2+ is 10−7M) and occupied at levels, which are reached upon stimulation (∼10−7M − 10−6M). This means that the affinity constants (Ka) for Ca2+ should be ∼10−6M−1.
The protein must show selectivity and preference for Ca2+ in the presence of other cations like Mg2+ and K+.
After binding to Ca2+, a Ca2+ sensor must undergo a conformational change that either alters its interaction with other molecules or changes its activity if it is an enzyme. Formation of Ca2+ protein complex is reversible.
The kinetics of interaction should be very fast so as to correspond with the short lived Ca2+ signature.
(b) EF Hand, Structure and Function.
Most of the Ca2+ sensors bind Ca2+ using a helix-loop-helix motif termed as the ‘EF hand’ or the elongation factor, which binds a single Ca2+ molecule with high affinity. The Ca2+ sensors utilize the side chain oxygen atoms of the EF hand motif for Ca2+ coordination. In 1973 Kretsinger and Nockolds37 first discovered the EF hand structural motif in the crystal structure of parvalbumin. The properties, structure and function of EF hands are described below.
The EF hand motifs mostly exist in pairs, which helps in the stabilization of the protein structure. Exceptions to this norm include proteins such as, parvalbumin and Plasmodium falciparum membrane surface protein. Frequently, EF hand pairs interact through antiparallel β-sheets, which allow cooperation in Ca2+ binding.
The EF hand is a highly conserved 29 amino-acid motif consisting of a α helix E (residue 1–10), a loop (residue 10–21), which binds the Ca2+ ion and a second α-helix F (residue 19–29) (Moews and Kretsinger, 1975). The loop consists of 12 residues with the pattern X (D), Y(D), Z (D), −Y (K), −X (D), −Z (E) which participate in binding Ca2+ and the intervening residues are represented by an asterisks (*). A representation of the EF hand and loop is shown in (Fig. 3A and B), respectivel.
The Ca2+ ion is coordinated by an oxygen atom or by a bridging water molecule (−x) of the side chains of residues 10 (X), 12 (Y), 14 (Z) and 18 (−X). The ligand at vertex (−Y) is the carbonyl oxygen at residue 16.38 Position 21 (−Z) is usually Glutamate and is the sixth residue to coordinate Ca2+.
In most of the functional EF hand motifs, the first amino acid is Aspartate (Asp) and the twelfth is Glutamate (Glu), Glu contributes both its side chain oxygen atoms to the metal ion coordination. Since there are 7 oxygen ligands, the Ca2+ coordination exhibits pentagonal bipyramidal symmetry, whose axis is 10(X)–18(−X). Glycine (Gly), present at the apex of the loop is an invariant residue and allows the loop to take a sharp bend. Each loop is flanked on either side by 8–10 residues of alternating hydrophilic and hydrophobic character. This arrangement favors the formation of the outer and inner surface of an alpha helical cylinder respectively.
Most of the EF hand proteins are characterized by the relatively high percentage of acidic residues (Troponin C, 29%, CaM, 25%, intestinal Ca2+ binding protein 23%, and parvalbumin, 18%). Several isoforms of an EF hand protein may exist in a single organism. The Ca2+ binding affinities of the EF hand protein vary substantially (Kd = 10−4−10−9M) and depend on the amino acid sequence of the protein, especially with regards to the 12 residue consensus loop that provide all the acids that directly ligate to Ca2+ ions. When Ca2+ binds to [Ca2+]cyt sensors their structural and/or enzymatic properties change and their subsequent interactions with target proteins can alter enzymatic activities, cytoskeletal orientation, protein phosphorylation cascades and gene expression. It is believed that these changes result in stress tolerance or a developmental switch.
Figure 3.
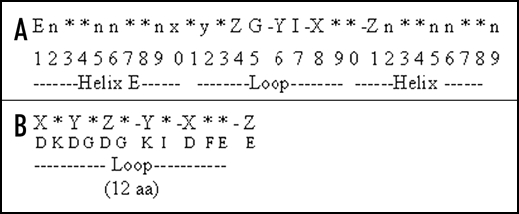
(A) Helix-loop-helix pattern of an EF hand. The EF hand is a highly conserved 29 amino-acid motif consisting of a α helix E (residue 1–10), a loop (residue 10–21), which binds the Ca2+ ion and a second α-helix F (residue 19–29). (B) The canonical EF hand consensus sequence of the 12 amino acid Ca2+ binding loop. The loop consists of 12 residues with the pattern X (D), Y(D), Z (D), -Y (K), -X (D), -Z (E) which participate in binding Ca2+ and the intervening residues are represented by an asterisks (*).
(c) Important calcium sensors in plants.
The major family of Ca2+ sensors includes; (1) Calmodulin (CaMs); (2) Calmodulin like proteins; (3) Calcium dependent protein kinases (CDPKs); (4) Calcineurin B-like proteins.
(1) Calmodulin: CaM.
Calmodulin (CaM) is a small (17 KDa), highly conserved, acidic protein with two globular domains each containing two EF hands connected by a flexible α-helical linker.39 CaM is found in the apoplast and in the cytosol, ER and the nucleus of plant cells. Within the cytosol, the estimated CaM concentration is 5 to 40 µM.40 Role of CaM has been implicated in many physiological processes like light, gravity, mechanical stress, phytohormones, pathogens, osmotic stress, heat shock and chilling.23,29,40
The structure of CaM was first solved by Babu et al., 1985, and revealed that all 4 EF hands are saturated by Ca2+ ions (4 Ca2+). CaM appears to be regulatory protein and induces large changes in inter-helical angles as Ca2+ is bound. The affinity of CaM for Ca2+ is influenced by the presence of particular target proteins.40 CaM can also regulate gene expression by binding to specific transcription factors.41
(2) CaM-like proteins.
Plants also possess CaM-like proteins, which differ from the CaM in containing more than 148 amino-acid residues and have between one to six EF hand motifs. They possess limited homology to CaM (75% identity with canonical CaM isoforms.39 In Arabidopsis, they include: CaBP-22, TCH2 and TCH3, AtCP1, NADPH oxidases, and Ca2+ binding protein phosphatases such as ABI 1 and ABI 2. These proteins have been implicated in cellular responses to diverse environmental, developmental and pathological challenges.
(3) Ca2+-dependent protein kinases.
5 types of Ca2+ regulated protein kinases have been reported in plants: (i) Ca2+ dependent and CaM independent protein kinases (CDPKs); (ii) CDPK-related protein kinases (CRKs); (iii) CaM dependent protein kinases (CaMKs); (iv) Ca2+/CaM-dependent protein kinases (CCaMK); (v) SOS3/CBL interacting protein kinases (SIPKs/CIPKs).
(i) Ca2+-dependent and CaM-independent protein kinases CDPKs.
The CDPKs are ubiquitous in plants. There are at least 34 genes encoding CDPKs in the Arabidopsis genome42 and similar numbers in other plant species. Apart from plants, CDPKs are also found in protozoans and algae. They generally have four EF hands at their C-terminus that bind Ca2+ and activate the serine/threonine kinase activity of the enzyme. These kinases require micromolar concentrations of Ca2+ for their activity and have no requirement of CaM or lipids. They have a unique structure as N-terminal protein kinase domain is fused with C-terminal auto-regulatory domain and a CaM like domain, which has Ca2+ binding EF hand or helix-loop-helix motif. The autoinhibitory domain of CDPKs is a 30 amino acid sequence, which acts as a pseudo-substrate.43 The N-terminal domain of CDPKs is variable and provides specificity to different CDPK isoforms. These enzymes show several fold stimulation with Ca2+ and show autophosphorylation. The binding of Ca2+ to some of CDPKs, is modulated by lipids or phosphorylation.42 Ca2+ binding to CDPK effects conformation of the kinase and relieve the inhibition caused by the autoinhibitory region.
CDPKs are implicated in pollen development, control of cell cycle, phytohormone signaling, light-regulated gene expression, gravitropism, thigmotropism, cold acclimation, salinity tolerance, drought tolerance and responses to pathogens.44
CDPK-related protein kinases (CRKs).
CRKs are similar to CDPKs expect that the CaM-like region is poorly conserved with degenerate or truncated EF hands that may not be able to bind Ca2+. There are at least seven CRKs in Arabidopsis genome, and orthologues of these are present in many plant species. However, the regulation and function of these kinases are not known.45
(iii) CaM kinases (CaMKs).
Several CaMKs have been cloned from Arabidopsis and other plants.46 Their kinase activity is stimulated by CaM dependent autophosphorylation and their catalytic activity is also modulated by CaM. They are highly expressed in rapidly growing cells and tissues of the root and flower.46
(iv) Ca2+/CaM-dependent protein kinases (CCaMKs).
These are a group of Ca2+-dependent kinases, which in addition to Ca2+ also requires CaM for their activity. Thus CaM besides acting directly could also exert its effect by binding to protein kinases and modulating their activity. A Ca2+/CaM-dependent protein kinase (CCaMK) was characterized from lily and other plant species.47 Sequence analysis revealed the presence of an N-terminal catalytic domain, a centrally located CaM-binding domain and a C-terminal visinin-like domain containing only three EF hands. Biochemical studies of CCaMK established that Ca2+ and CaM stimulates CCaMK activity. In the absence of CaM, Ca2+ promotes autophosphorylation of CCaMK. The phosphorylated form of CCaMK possesses more kinase activity than the non-phosphorylated form.
(v) SOS3/CBL interacting protein kinases (SIPKs/CIPKs).
Calcineurin B-like proteins were found to interact specifically with a class of serine-threonine protein kinases known as CBL interacting protein kinases (CIPKs).1, 2 Recently, a novel CIPK from pea has been reported and found to interact and phosphorylate the pea.48
(4) Calcineurin B-like proteins.
Calcineurin B-like proteins (CBLs) are relatively a new class of calcium sensors discovered in Arabidopsis originally, in search for the genes imparting salt tolerance and maintaining cellular ion homeostasis.49, 50 Molecular analysis of the sos mutants opened a new chapter in relation to salt stress signalling that led to the discovery of a pathway that transduce a salt stress induced Ca2+ signal to reinstate cellular ion homeostasis. Currently 10 CBL and 25 CBL-interacting protein kinases (CIPKs) have been reported from Arabidopsis.2 The CBL-CIPK network is also widely distributed among higher plants but except Arabidopsis, the complexity and characterization of this pathway remains largely unrevealed. As different plants vary in their genome complexity, phenotype and physiology therefore, specie specific function or functional diversification can be expected. The essential role imparted by CBL-CIPK genes in stress tolerance, necessitates their detailed characterization from higher plants. In fact, there has been no report on experimental characterization of CBL from any higher plant except Arabidopsis. AtCBL3, in particular has been largely overlooked even in Arabidopsis. Recently, we (Mahajan et al., 2006)48 have reported the cloning and characterization of a novel CIPK and its interacting partner CBL from pea. Pea CIPK showed autophosphorylation and could phosphorylate pea CBL. Both pea CBL and CIPK were found to be coordinately upregulated in response to various stresses such as cold, salinity but not to dehydation stress.48
(d) Ca2+-binding proteins without EF hands.
There are several proteins that bind Ca2+ but do not contain EF hand motifs. These include the phospholipase D (PLD), annexins, calreticulin and Pistil-expressed Ca2+ binding protein (PCP).
Phospholipase D
The activity of PLD, which cleaves membrane phospholipids into a soluble head group and PA, is regulated by [Ca2+]cyt through a Ca2+/phospholipids binding-site termed as the ‘C2 domain’.51 PLD activity is implicated in cellular responses to ethylene and ABA, α amylase synthesis in aleurone cells, stomatal closure, pathogen responses, leaf senescence and drought tolerance.51 Plants posses several PLD isoforms that differ in their affinity for Ca2+ and their modulation by phosphoinositides, free fatty acids and lysolipids.51 These biochemical modulators of PLD activity are the substrates or products of PLC, which generates IP3, DAG, phospholipase A2 and DAG-kinase, both of which are regulated by CaM. It is suggested that [Ca2+]cyt signaling cascades might coordinate the activities of these diverse enzymes to effect specific responses to the environmental stimuli.52
Annexins
These are a family of proteins in plants and animals that bind phospholipids in a Ca2+ dependent manner and contain four to eight repeats of approximately 70 amino acids.53 Although exact function of annexins is not known, plant annexins are implicated in secretory processes and some have ATPase, peroxidase activities.
Calreticulin
It is a Ca2+ sequestering protein in the ER and functions as a molecular chaperone.54 The function of calreticulin is also implicated in Ca2+ homeostasis.55
Pistil expressed Ca2+-Binding Protein (PCP)
A 19 kDa novel Ca2+ binding protein (PCP) expressed in anthers and pistil was isolated.56 PCP is high capacity (binds 20 mol of Ca2+ per mol of PCP), low affinity Ca2+ binding protein. Role of PCP has been implicated in pollen-pistil interactions and/or pollen development.
Acknowledgements
I thank Dr. Renu Tuteja for critical reading and corrections on the article. This work was partially supported by the grants from the Department of Biotechnology (DBT), Department of Science and Technology (DST) and Defence Research and Development Organisation (DRDO), Government of India. I apologize if some references could not be cited due to space constraint.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=4176
References
- 1.Mahajan S, Tuteja N. Cold, salinity and drought stresses: an overview. Arch Biochem Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan S, Sopoy SK, Tuteja N. CBL-CIPK paradigm: role in calcium and stress signaling in plants. Proc Indian Nat Sci Acad. 2006;72:63–78. [Google Scholar]
- 3.Xiong TC, Bourque S, Lecourieux D, Amelot N, Grat S, Briere C, Mazars C, Pugin A, Ranjeva R. Calcium signaling in plant cell organelles delimited by a double membrane. Biochim Biophys Acta. 2006;1763:1209–1215. doi: 10.1016/j.bbamcr.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan B, Davydov O, Knight H, Galon Y, Knight MR, Fluhr R, Fromm H. Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. Plant Cell. 2006;18:2733–2748. doi: 10.1105/tpc.106.042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harada A, Shimazaki KI. Phototropins and blue light-dependent calcium signaling in higher plants. Photochem Photobiol. 2006 Mar 1; doi: 10.1562/2006-03-08-IR-837. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Noel G, Tognetti J, Nagaraj V, Wiemken A, Pontis H. Calcium is essential for fructan synthesis induction mediated by sucrose in wheat. Planta. 2006;225:183–191. doi: 10.1007/s00425-006-0339-4. [DOI] [PubMed] [Google Scholar]
- 7.Muller JF, Hulster AA, Papke OC, Ball MC, Marschner H. Transfer of PCDD/PCDF from contaminated soils into carrots, lettuce and peas. Chemosphere. 1994;29:2175–2181. doi: 10.1016/0045-6535(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 8.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstrom HG. Calcium and plant growth. Biol Rev. 1968;43:287–316. [Google Scholar]
- 10.Bush DS. Calcium regulation in plant cells and its role in signaling. Ann Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- 11.Marschner H. Mineral Nutrition of Higher Plants. London: Academic Press; 1986. pp. 252–254. [Google Scholar]
- 12.Loneragan JF, Snowball K. Calcium requirements of plants. Aust J Agric Res. 1969;20:465–478. [Google Scholar]
- 13.Hirschi K. Vacuolar H+/Ca2+ transport: who's directing the traffic? Trends Plant Sci. 2001;6:100–104. doi: 10.1016/s1360-1385(00)01863-x. [DOI] [PubMed] [Google Scholar]
- 14.Harper JF. Dissecting calcium oscillators in plant cells. Trends Plant Sci. 2001;6:395–397. doi: 10.1016/s1360-1385(01)02023-4. [DOI] [PubMed] [Google Scholar]
- 15.Denis V, Cyert MS. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol. 2002;156:29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Axelsen KB, Palmgren MG. Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol. 2001;126:696–706. doi: 10.1104/pp.126.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang I, Sze H, Harper JF. A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proc Nat Acad Sci USA. 2000;97:6224–6229. doi: 10.1073/pnas.97.11.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasi-Caldogno F, Carnelli A, DeMichelis MI. Identification of the plasma membrane Ca2+ ATPase and of its autoinhibitory domain. Plant Physiol. 1995;108:105–113. doi: 10.1104/pp.108.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garciadeblas B, Benito B, Rodrèguez-Navarro A. Plant cells express several stress calcium ATPases but apparently no sodium ATPase. Plant Soil. 2001;235:81–192. [Google Scholar]
- 20.Hirschi KD, Zhen RG, Cunningham KW, Rea PA, Fink GR. CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA. 1996;93:8782–8786. doi: 10.1073/pnas.93.16.8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chanson A. Characterization of the tonoplast Ca2+/H+ antiport system from maize roots. Plant Physiol Biochem. 1994;32:341–346. [Google Scholar]
- 22.Macklon AES. Calcium fluxes at plasmalemma and tonoplast. Plant Cell Environ. 1984;7:407–413. [Google Scholar]
- 23.Rudd JJ, Franklin-Tong VE. Unravelling response-specificity in Ca2+ signalling pathways in plant cells. New Phytol. 2001;151:7–33. doi: 10.1046/j.1469-8137.2001.00173.x. [DOI] [PubMed] [Google Scholar]
- 24.Trewavas A. Le calcium, c'est la vie: calcium makes waves. Plant Physiol. 1999;120:1–6. doi: 10.1104/pp.120.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drøbak BK, Watkins PAC. Inositol (1,4,5) trisphosphate production in plant cells: an early response to salinity and hyperosmotic stress. FEBS Lett. 2000;481:240–244. doi: 10.1016/s0014-5793(00)01941-4. [DOI] [PubMed] [Google Scholar]
- 26.Franklin-Tong VE, Holdaway-Clarke TL, Straatman KR, Kunkel JG, Hepler PK. Involvement of extracellular calcium influx in the self-incompatibility response of Papaver rhoeas. Plant J. 2002;29:333–345. doi: 10.1046/j.1365-313x.2002.01219.x. [DOI] [PubMed] [Google Scholar]
- 27.Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 1999;19:735–747. doi: 10.1046/j.1365-313x.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- 29.Fasano JM, Massa GD, Gilroy S. Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul. 2002;21:71–88. doi: 10.1007/s003440010049. [DOI] [PubMed] [Google Scholar]
- 30.Moore CA, Bowen HC, Scrase-Field S, Knight MR, White PJ. The deposition of suberin lamellae determines the magnitude of cytosolic Ca2+ elevations in root endodermal cells subjected to cooling. Plant J. 2002;30:457–466. doi: 10.1046/j.1365-313x.2002.01306.x. [DOI] [PubMed] [Google Scholar]
- 31.Knight MR, Read ND, Campbell AK, Trewavas AJ. Imaging calcium dynamics in living plants using semisynthetic recombinant aequorins. J Cell Biol. 1993;12:83–90. doi: 10.1083/jcb.121.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell AK, Trewavas AJ, Knight MR. Calcium imaging shows differential sensitivity to cooloing and communication in luminous transgenic plants. Cell Calcium. 1996;19:211–218. doi: 10.1016/s0143-4160(96)90022-6. [DOI] [PubMed] [Google Scholar]
- 33.Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. Calcium mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;6:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- 34.Knight H, Trewavas AJ, Knight MR. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blume B, Nürnberger T, Nass N, Scheel D. Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell. 2000;12:1425–1440. doi: 10.1105/tpc.12.8.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price AH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR. Oxidative signals in tobacco increase cytosolic calcium. Plant Cell. 1994;6:1301–1310. doi: 10.1105/tpc.6.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kretsinger RH, Nockolds CE. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973;248:3313–3326. [PubMed] [Google Scholar]
- 38.Strynadka NCJ, James MNG. Crystal structures of the helix-loop-helix calcium-binding proteins. Ann Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- 39.Luan S, Kudla J, Rodrèguez-Concepción M, Yalovsky S, Gruissem W. Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell. 2002;14:S389–S400. doi: 10.1105/tpc.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zielinski RE. Calmodulin and calmodulin-binding proteins in plants. Ann Rev Plant Physiol Plant Mol Biol. 1998;49:697–725. doi: 10.1146/annurev.arplant.49.1.697. [DOI] [PubMed] [Google Scholar]
- 41.Bouché N, Scharlat A, Snedden W, Bouchez D, Fromm H. A novel family of calmodulin-binding transcription activators in multicellular organisms. J Biol Chem. 2002;277:21851–21861. doi: 10.1074/jbc.M200268200. [DOI] [PubMed] [Google Scholar]
- 42.Cheng SH, Willmann MR, Chen HC, Sheen J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002;129:469–485. doi: 10.1104/pp.005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harper JF, Huang JF, Lloyd SJ. Genetic identification of an auto inhibitor in CDPK, a protein kinase with a calmodulin like domain. Biochemistry. 1994;33:7278–7287. doi: 10.1021/bi00189a031. [DOI] [PubMed] [Google Scholar]
- 44.Xiong L, Schumaker KS, Zhu JK. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002;14:S165–S183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harmon AC, Gribskov M, Gubrium E, Harper JF. The CDPK superfamily of protein kinases. New Phytol. 2001;151:175–183. doi: 10.1046/j.1469-8137.2001.00171.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Lu YT. Calmodulin-binding protein kinases in plants. Trends Plant Sci. 2003;8:123–127. doi: 10.1016/S1360-1385(03)00013-X. [DOI] [PubMed] [Google Scholar]
- 47.Lu YT, Hidaka H, Feldman LJ. Characterization of a calcium/calmodulin protein kinase homologue from maize roots showing light-regulated gravitropism. Planta. 1996;199:18–24. doi: 10.1007/BF00196876. [DOI] [PubMed] [Google Scholar]
- 48.Mahajan S, Sopoy SK, Tuteja N. Cloning and characterization of CBL-CIPK signaling components from a legume (Pisum sativum) FEBS J. 2006;273:907–925. doi: 10.1111/j.1742-4658.2006.05111.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Zhu JK. A calcium sensor homolog required for plant salt tolerance. Science. 1998;280:1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- 50.Kudla J, Xu Q, Harter K, Gruissem W, Luan S. Genes for calcineurin B-like proteins in Arabidopsis are diferentially regulated by stress signals. Proc Natl Acad Sci USA. 1999;96:4718–4723. doi: 10.1073/pnas.96.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X. Plant phospholipases. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:211–231. doi: 10.1146/annurev.arplant.52.1.211. [DOI] [PubMed] [Google Scholar]
- 52.Ritchie SM, Swanson SJ, Gilroy S. From common signalling components to cell specific responses: insights from the cereal aleurone. Physiol Plant. 2002;115:342–351. doi: 10.1034/j.1399-3054.2002.1150303.x. [DOI] [PubMed] [Google Scholar]
- 53.Clark GB, Roux SJ. Annexins of plant cells. Plant Physiol. 1995:1133–1139. doi: 10.1104/pp.109.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baluška F, Šamaj J, Napier R, Volkmann D. Maize calreticulin localizes preferentially to plasmodesmata in root apex. Plant J. 1999;19:481–488. doi: 10.1046/j.1365-313x.1999.00530.x. [DOI] [PubMed] [Google Scholar]
- 55.Michalak M, Mariani P, Opas M. Calreticulin, a multifunctional Ca2+ binding chaperone of the endoplasmic reticulum. Biochem Cell Biol. 1998;76:779–785. doi: 10.1139/bcb-76-5-779. [DOI] [PubMed] [Google Scholar]
- 56.Furuyama T, Dzelzkalns VA. A novel calcium-binding protein is expressed in Brassica pistils and anthers late in flower development. Plant Mol Biol. 1999;39:729–737. doi: 10.1023/a:1006169808171. [DOI] [PubMed] [Google Scholar]


