Abstract
The eukaryotic LIM domain defines a double zinc-finger like structure that functions as a protein-protein interaction module. Whereas in animals the LIM domain is found in numerous proteins of diverse functions, plants possess only a limited number of LIM domain-containing proteins (LIMs). It is noteworthy that most of plant LIMs belong to a same family that is structurally related to the animal Cysteine-Rich Proteins (CRPs). In the September issue of The Plant Cell, we have provided evidence that the tobacco WLIM1 is able to bind actin filaments in a direct manner, to stabilize them and to trigger actin bundling both in vitro and in vivo. These data, together with recent reports on animal CRPs, strongly suggest that these proteins represent a novel class of actin cytoskeleton regulators. In this addendum, we give a brief history of the research that has been conducted on plant LIMs in our lab. Additionally, we show that the GFP-fused tobacco WLIM1 protein is able to properly localize when ectopically expressed in monkey Vero cells, indicating that, despite a relatively low degree of identity/similarity, animal CRPs and plant LIMs display a very similar actin binding activity.
Key Words: actin-binding proteins, actin cytoskeleton, actin bundling, cysteine-rich proteins, LIM proteins
While the first description of a plant LIM domain protein dates back to 1992, it was only in the past few years that evidence for an association of plant LIMs with actin-based structures was gradually obtained.4 Among the first difficulties encountered was the choice of the model plant. Sunflower, which was the first species from which a LIM protein-coding sequence was isolated, for its recalcitrance to transformation turned out not to be suitable for functional studies.4 Although immunocytological data, obtained using two specific monoclonal antibodies, revealed an accumulation of the sunflower pollen protein PLIM1 in the F-actin enriched germination cones of the mature pollen grains, no direct evidence for actin binding could be observed, neither in fixed sunflower pollen grains, nor in in vitro overlay assays.5 A second hint that plant LIMs could participate in actin-based functions was obtained with another sunflower protein, WLIM1, which was found to accumulate along filamentous structures in the phragmoplast region of dividing integumental cells of the sunflower ovary.6 These studies also provided clear evidence not only for a cytoplasmic, but also for a nuclear localisation of this protein and these cytoplasmic and nuclear functions appeared to be separated in a tissue-dependent manner: as an example, in integumental cells of the ovary the protein was predominantly found in the nucleus, while in the cells surrounding the embryo sac it appeared exclusively cytoplasmic. Switching to tobacco, an easily transformable plant species, as a new model system for these studies did not immediately meet the expected success either. In this case the failure to generate transgenic plants expressing a tagged tobacco LIM protein was finally ascribed to a lethal effect of its overexpression using the constitutive CaMV35S promoter. Numerous transformation attempts were undertaken as well on BY2 cells as on tobacco leaf discs but no experiment went beyond the callus stage where the cells died. It was finally the use of an inducible promoter construct (pTA7002) that led to a successful generation of transformed BY2 cell lines. These cells, when transformed with the GFP fusion construct of the WLIM1 protein, displayed fluorescent filaments which were identified as actin-based structures using colabeling experiments with rhodamine-phalloidin and cytoskeleton-interfering drugs.
Plant LIMs are related to the animal CRPs in their size and domain organization: they both have a short N-terminus, two 52 residue-long LIM domains separated by a 45–55 residue spacer, and generally a short C-terminus. Sequence homology however is low, as they share only about 30% of identical residues. This raises the question as to whether the plant LIMs and the animal CRPs are functional homologues or not. Heterologous expression of the tobacco WLIM1 protein in simian Vero cells showed that the plant protein binds to filamentous structures identified as F-actin-containing stress fibers, with thickened ends corresponding to focal adhesions (Fig. 1). This pattern exactly reflects that of the CRPs as well as that of zyxin, a non-CRP-type LIM domain protein which appears to be specific to animal cells and which has also been identified in focal adhesions and along stress fibers.7 The binding of the tobacco protein to the animal actin cytoskeleton is another indication for a direct association of this protein with actin, and for the conservation of actin binding sites in the plant LIMs and the animal CRPs. The animal CRP1 was found not only to bind to, but also to bundle actin filaments, as does the tobacco WLIM1 protein.8 Is it therefore possible that all the members of the CRP/plant LIM protein family are F-actin binding and bundling proteins? Experiments in progress in our laboratory on other plant LIMs suggest that the actin binding properties are not necessarily conserved in all of them: for instance, the protein WLIM2 from tobacco, which shares 52% identity (+12% sequence similarity) with WLIM1, does not exhibit a clear association with the actin cytoskeleton neither in undifferentiated BY2 cells nor in differentiated leaf epidermal cells from N. benthamiana (Gatti S, unpublished results), very clearly indicating that the (nuclear and/or cytoplasmic) functions of the various LIM proteins can diverge at least partially.
Figure 1.
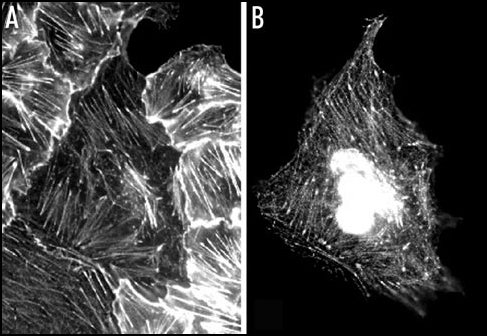
Colocalization of the ectopically-expressed tobacco WLIM1 protein and actin filaments in monkey Vero cell. (A) Actin cytoskeleton as visualized by rhodamine-phalloidin staining. (B) Fluorescent pattern observed for the GFP-fused WLIM1 protein.
The use of the now classical approaches for functional studies, e.g., insertion mutant phenotype analysis and identification of protein partners (other than actin), should provide a better insight into the various functions, cytoplasmic as well as nuclear, of the different members of the LIM family in plants.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3614
References
- 1.Thomas C, Hoffmann C, Dieterle M, Van Troys M, Ampe C, Steinmetz A. Tobacco WLIM1 is a novel F-actin binding protein involved in actin cytoskeleton remodeling. Plant Cell. 2006;18:2194–2206. doi: 10.1105/tpc.106.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang S, Robinson RC, Gao LY, Matsumoto T, Brunet A, Blanchoin L, Staiger CJ. Arabidopsis VILLIN1 generates actin filament cables that are resistant to depolymerization. Plant Cell. 2005;17:486–501. doi: 10.1105/tpc.104.028555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovar DR, Staiger CJ, Weaver EA, McCurdy DW. AtFim1 is an actin filament crosslinking protein from Arabidopsis thaliana. Plant J. 2000;24:625–636. doi: 10.1046/j.1365-313x.2000.00907.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheung AY, Wu HM. Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell. 2004;16:257–269. doi: 10.1105/tpc.016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltz R, Schmit AC, Kohnen M, Hentges F, Steinmetz A. Differential localization of the LIM domain protein PLIM-1 in microspores and mature pollen grains from sunflower. Sex Plant Reprod. 1999;12:60–65. [Google Scholar]
- 6.Mundel C, Baltz R, Eliasson A, Bronner R, Grass N, Krauter R, Evrard JL, Steinmetz A. A LIM-domain protein from sunflower is localized to the cytoplasm and/or nucleus in a wide variety of tissues and is associated with the phragmoplast in dividing cells. Plant Mol Biol. 2000;42:291–302. doi: 10.1023/a:1006333611189. [DOI] [PubMed] [Google Scholar]
- 7.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran TC, Singleton C, Fraley TS, Greenwood JA. Cysteine-rich protein 1 (CRP1) regulates actin filament bundling. BMC Cell Biol. 2005:6–45. doi: 10.1186/1471-2121-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]


