Abstract
Light gradients in the soil have largely been overlooked in understanding plant responses to the environment. However, roots contain photoreceptors that may receive ambient light through the soil or piped light through the vascular cylinder. In recent experiments we demonstrated linkages between phototropin-1 photoreceptor production, root growth efficiency, and drought tolerance, suggesting that root plasticity in response to light signals contributes to the ecological niche of A. thaliana. However, the availability of light cues in natural soil environments is poorly understood, raising questions about the relevance of light-mediated root growth for fitness in nature. Additionally, photoreceptor expression is characterized by pleiotropy so unique functions cannot be clearly ascribed to root vs. shoot sensory mechanisms. These considerations show that challenges exist for resolving the contribution of light-sensing by roots to plant adaptation. We suggest that blue-light sensing in roots of A. thaliana provides a model system for addressing these challenges. By calibrating blue light gradients in soils of diverse A. thaliana habitats and comparing fitness of phot1 mutant and wild-type controls when grown in presence or absence of soil light cues, it should be possible to elucidate the ecological significance of light-mediated plasticity in roots.
Key Words: phototropin, roots, drought-tolerance, photoreceptors, Arabidopsis thaliana
In plants, the capacity to sense and respond to variation in light quality is exploited in ecological interactions with neighbors,1 in optimizing light interception for photosynthesis,2 and even in collecting heat as a reward for insect pollinators in cold environments.3 Perhaps because the physiological and developmental functions modified by these light responses are readily observed in above ground organs (leaves, stems, flowers, etc.) light sensing and its adaptive significance belowground have largely been ignored. Light gradients underground are commonly considered redundant in information content to gravity, based on the similar directional responses of root growth to the two stimuli. This premise assumes that roots do not respond to light gradients established by vegetative canopies, or to light mosaics created in heterogeneous soils. However, such assumptions are problematic on several grounds—light piping by vascular elements makes the air-soil interface less of a barrier than a filter for light signals,4 even in uniform soil, the attenuation of light with depth informs the root of its position relative to the surface in a way that gravity cannot; and natural selection has favored a role for photo-sensory systems in other underground processes (e.g., phytochrome-mediated seed germination, ref. 1) suggesting that light signals in the soil can provide valuable indicators of environmental conditions for growth and development.
Because root growth responds to hormonal and ionic gradients established by signal reception in leaves,5 one might argue that photoreceptors in the roots themselves are redundant or at best, relatively unimportant. Yet photoreceptors provide unique information when deployed in different locations. For example, the quality of light intercepted at the leaf or stem allows for rapid reallocation of resources from root to shoot system in relation to crowding by vertically oriented foliage (e.g., during shade avoidance, ref. 1), but may be less effective at directing responses to soil disturbance, desiccation or rosette density.
In new research on the blue light photoreceptor phototropin-1 we show that the abundance of the photoreceptor in roots correlates with enhanced root growth efficiency. Mutants lacking the phot1 protein exhibit comparably random root growth and lower desiccation tolerance, suggesting that natural selection may have acted on root-mediated light sensing to improve drought tolerance in A. thaliana. Demonstrating that plastic responses of roots to soil light stimuli contribute to drought tolerance in the wild will require new research that characterizes underground light environments in natural habitats and measures selection on light-sensing in roots independent of pleiotropic effects on above-ground (leaf and stem) functions. We review our findings on blue-light mediated plasticity in root growth of A. thaliana, and propose that genetic polymorphism in Arabidopsis phototropin-1 provides a model system for addressing the adaptive significance of root photo-sensory systems in nature.
Arabidopsis thaliana mutant plants lacking the blue light photoreceptor, phototropin-1, exhibit significantly reduced drought tolerance compared to the wild type background COL- O genotype for the phot1 mutation. Under dry (but not wet) soil conditions, wild type plants grow twice as large as phot1 mutants and plant size is highly correlated with root growth efficiency, the capacity of roots to grow directly away from the soil surface toward a belowground water supply. Using a translationally-fused phot1-gfp (green fluorescent protein) gene-construct to localize protein expression in roots, we found that high root growth efficiency is primarily limited to shallow rooting zones where soil drying is most rapid and phot1 protein most concentrated. This pattern suggests a role of phot1 in promoting efficient root growth by cueing roots to their proximity to the soil surface. However, if this conclusion is correct then blue light must attain sufficient intensity in natural soils to activate root-localized phot1 and pleiotropic effects in the shoot system cannot solely explain the impact of phot1 on drought tolerance.
Light Goes Underground
Actual light mosaics in the soil are not known for any natural habitat, and are likely to vary temporally as well as spatially with precipitation, erosion, frost-heave and other forms of disturbance. Characterizing this heterogeneity is necessary to understand whether underground light signals are sufficiently strong to drive photoreceptor pathways and, if so, to elucidate possible relationships between soil light cues and ecological factors (disturbance, exposure, desiccation, etc.) regulating plant growth and fitness. Phot1 has two features that make its activation in the soil probable: an exquisite sensitivity to light of very low fluence rate7 and strong effects on growth and survival during seedling establishment when roots are closest to the soil surface.8 Root localized photoreceptors may receive light from two possible sources: ambient light filtering through the soil matrix9 and supplemental light piped downward and leaking outward from the xylem.4 Ambient light transmission to roots depends on soil color and particle size10: less than 0.005% of surface light penetrates more than 4 mm through black and brown soils. However in lighter (gray-white) soils, light transmission depth more than doubles.11 Light moves further through coarse soils than fine ones.10 Light also leaks out of xylem elements that act as fiber optic cables in the vascular cylinder, further enhancing its transmission underground. For example, propagation through woody xylem could more than double light transmission distance in the soil.12 Short wavelengths attenuate most rapidly for both ambient and piped light signals, implying that light becomes increasingly far red13 with soil depth.9,12 These trends in the laboratory represent “best case scenarios” for light transmission in nature where gravel, leaf litter, and other forms of organic debris may further diminish light penetration.8
Multi-Tasking Photoreceptors
All plant photoreceptors are pleiotropic, controlling multiple events during growth and development.13 Photoreceptor pleiotropy elicits physiological and developmental events in separate organs to coordinate functionally related modifications of plant form. Positive shoot phototropism, negative root phototropism, stomatal opening, and chloroplast relocation are all mediated by phot1.13 Since drought stress depends on the balance between transpiration and water uptake, pleiotropic effects of phot1 on these processes may act synergistically to influence desiccation tolerance (Fig. 1). In one model, consistent with our current understanding of phot1 function, phot1 mediated root phototropism reduces desiccation stress by facilitating root movement to deeper, wetter soil layers (Fig. 1B). According to this scenario, activation of phot1 in the roots maintains water flow to the shoot system allowing for enhanced leaf transpiration and stomatal opening (feed-forward control). Consistent with this idea, phot1 mutant plants exhibit a 35% reduction in transpiration rate compared to wild-type. Alternatively, phot1 activation in leaves may enhance stomatal opening, promoting transpiration and, in turn, increasing root elongation rate (Fig. 1A). Distinguishing between these causal pathways is key to understanding the importance of root-localized phot1 for desiccation tolerance.
Figure 1.
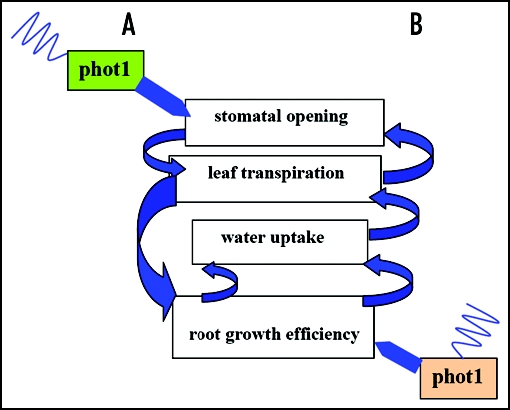
Alternative causal models for impact of phototropin-1 on drought tolerance in Arabidopsis thaliana. (A) Phot1 in leaves activates an increase in water loss via stomatal opening signaling roots to grow deeper: this enhances water uptake indirectly. (B) Phot1 in roots activiates root growth, directly enhancing water uptake.
Several strategies exist for controlling pleiotropy in root vs. shoot-localized phot1 expression. Soil amendments can be used to selectively to absorb (or transmit) blue light. If root growth in wild type plants resembles that of phot1 mutant plants when blue light cues are removed from the soil, but not from the aerial environment and this in turn aggravates desiccation stress, then results would corroborate the importance of light sensing by roots for drought tolerance. The main limitation of this technique is that it cannot be applied in natural soil habitats. More powerful approaches involve grafting15 or use of organ specific promoters to drive phot1 expression independently for root and shoot systems. With these genetic techniques, tests for a fitness impact of light sensing by roots can be conducted under natural soil environments. Development of these tools is in progress and we are confident that more light will soon be shed on the adaptive significance of root photo-sensory mechanisms for plant adaptation to the environment.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3638
References
- 1.Smith H. Physiological and ecological function within the phytochrome family. Ann Rev Plant Physiol Plant Mol Biol. 1995;46:289–315. [Google Scholar]
- 2.Ehleringer JR, Forseth I. Solar tracking by plants. Science. 1980;210:1994–1998. doi: 10.1126/science.210.4474.1094. [DOI] [PubMed] [Google Scholar]
- 3.Stanton ML, Galen C. Blue light controls solar tracking by flowers of an alpine plant. Plant Cell Environ. 1993;16:983–989. [Google Scholar]
- 4.Mandoli DF, Briggs WR. Fiber optics in plants. Sci Am. 1984;251:90–98. [Google Scholar]
- 5.Beveridge C. The ups and downs of signalling between root and shoot. New Phytol. 2000;147:413–416. doi: 10.1046/j.1469-8137.2000.00729.x. [DOI] [PubMed] [Google Scholar]
- 6.Galen C, Rabenold JJ, Liscum E. Functional ecology of a blue-light photoreceptor: Effects of phototropin-1 on root growth enhance drought tolerance in Arabidopsis thaliana. New Phytol. 2007;173:91–99. doi: 10.1111/j.1469-8137.2006.01893.x. [DOI] [PubMed] [Google Scholar]
- 7.Sakai T, Kagawa T, Kashara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. Nph1 and npl1: Blue light photoreceptors that mediate both phototropism and chloroplast relocation in Arabidopsis. PNAS USA. 2001;98:6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galen C, Huddle J, Liscum E. An experimental test of the adaptive evolution of phototropins: Blue-light photoreceptors controlling phototropism in Arabidopsis thaliana. Evolution. 2004;58:515–523. [PubMed] [Google Scholar]
- 9.Mandoli DF, Ford GAI, Waldron IJ, Nemson JA, Briggs WR. Some spectral properties of several soil types: Implications for photomorphogenesis. Plant Cell Environ. 1990;13:287–294. [Google Scholar]
- 10.Tester M, Morris C. The penetration of light through soil. Plant Cell Environ. 1987;10:281–286. [Google Scholar]
- 11.Kasperbauer MJ, Hunt PG. Biological and photometric measurement of light transmission through soils of various colors. Bot Gaz. 1988;149:361–364. [Google Scholar]
- 12.Sun Q, Yoda K, Suzuki M, Suzuki H. Vascular tissue in the stem and roots of woody plants can conduct light. J Exp Bot. 2003;54:1627–1635. doi: 10.1093/jxb/erg167. [DOI] [PubMed] [Google Scholar]
- 13.Briggs WR, Olney MA. Photoreceptors in plant morphogenesis to date: Five phytochromes, two cryptochromes, one phototropin and one superchrome. Plant Physiol. 2001;125:85–88. doi: 10.1104/pp.125.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Weele CM, Spollen WG, Sharp RE, Baskin TI. Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J Exp Bot. 2000;51:1555–1562. doi: 10.1093/jexbot/51.350.1555. [DOI] [PubMed] [Google Scholar]
- 15.Turnbull CGN, Booker JP, Leyser HMO. Micrografting techniques for testing long-distance signaling in Arabidopsis. Plant J. 2002;32:255–262. doi: 10.1046/j.1365-313x.2002.01419.x. [DOI] [PubMed] [Google Scholar]


