Abstract
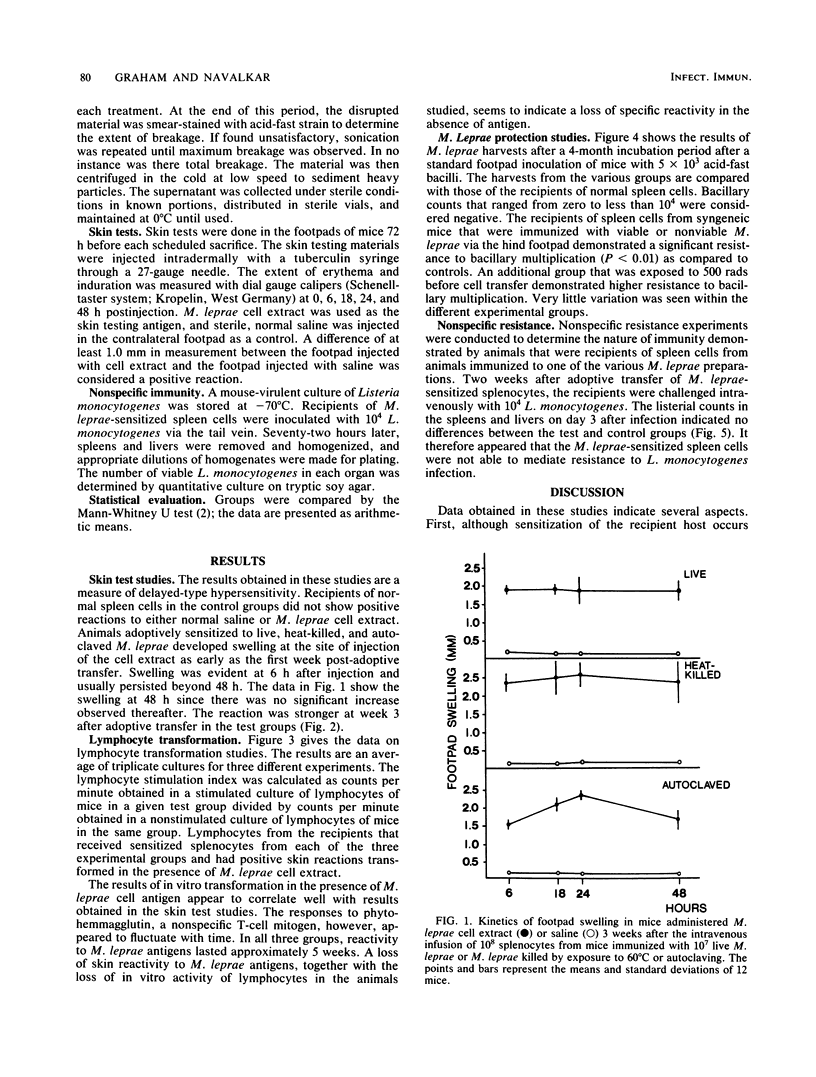
The immune response of mice to live, heat-killed, or autoclaved Mycobacterium leprae was investigated. After sensitization with 10(7) organisms in each group, recipient mice were transfused with the sensitized splenocytes 28 days later. A selected number of these mice were infected with 5 X 10(3) M. leprae, and the remaining animals were sacrificed at scheduled intervals for evidence of cell-mediated immunity to the M. leprae cell extract. Data from these and the bacteriological assays showed that all three materials induce cell-mediated immunity and also extend protection against the M. leprae challenge but not against a Listeria monocytogenes challenge. Adoptive immunity against M. leprae was expressed equally effectively in both non-irradiated animals and those sublethally (500 R) irradiated. This study reveals that, after adoptive transfer of immunity, a bacillary restriction occurs with concomitant onset of delayed hypersensitivity and that the protection observed could be specifically directed against an M. leprae challenge.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Zembala M. Contact sensitivity in the mouse. IV. The role of lymphocytes and macrophages in passive transfer and the mechanism of their interaction. J Exp Med. 1970 Jul 1;132(1):1–15. doi: 10.1084/jem.132.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada F. Quantitative studies of the adoptive immunological memory in mice. I. An age-dependent barrier to syngeneic transplantation. J Exp Med. 1966 Jul 1;124(1):1–14. doi: 10.1084/jem.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada F. The cellular basis of immunologic memory. Prog Allergy. 1971;15:223–267. [PubMed] [Google Scholar]
- Kirchner H., Chused T. M., Herberman R. B., Holden H. T., Lavrin D. H. Evidence of suppressor cell activity in spleens of mice bearing primary tumors induced by Moloney sarcoma virus. J Exp Med. 1974 Jun 1;139(6):1473–1487. doi: 10.1084/jem.139.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Transfer of adoptive immunity to tuberculosis in mice. Infect Immun. 1975 Jun;11(6):1174–1181. doi: 10.1128/iai.11.6.1174-1181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METAXAS M. N., METAXAS-BUEHLER M. Studies on the cellular transfer of tuberculin sensitivity in the guinea pig. J Immunol. 1955 Nov;75(5):333–347. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Berche P. A., Newborg M. F. Immunologic consequences of antibiotic-induced abridgement of bacterial infection: effect on generation and loss of protective T cells and level of immunologic memory. J Immunol. 1981 Jul;127(1):342–346. [PubMed] [Google Scholar]
- Reed S. G. Adoptive transfer of resistance to acute Trypanosoma cruzi infection with T-lymphocyte-enriched spleen cells. Infect Immun. 1980 May;28(2):404–410. doi: 10.1128/iai.28.2.404-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C. Vaccination of mice against M. leprae infection. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):222–226. [PubMed] [Google Scholar]
- Shepard C. C., Walker L. L., van Landingham R. Heat stability of Mycobacterium leprae immunogenicity. Infect Immun. 1978 Oct;22(1):87–93. doi: 10.1128/iai.22.1.87-93.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



