Abstract
Today's major excitement in biology centers on signaling: How can a cell or organism measure the myriad of environmental cues, integrate it, and acclimate to the new conditions? Hormonal signals and second messengers are in the focus of most of these studies, e.g., regulation of glucose transporter GLUT4 cycling by insulin, or regulation of plant growth by auxin or brassinosteroids.1–3 In comparison, we generally assume that we know almost everything about basic metabolism since it has been studied for many decades; for example we know since the early 80s that allosteric regulation by fructose-2,6-bisphophate plays an important role in regulating glycolysis in plants and animals.4 This may be the reason why studies of metabolism appear to be a bit out of fashion. But if we look to other organisms such as E. coli or yeast, we rapidly realize that metabolism is controlled by complex interconnected signaling networks, and that we understand little of these signaling networks in humans and plants.5,6 As it turns out, the cell registers many metabolites, and flux through the pathways is regulated using complex signaling networks that involve calcium as well as hormones.
Key Words: flux, fluxome, glucose, glutamate, phosphate, sucrose, fluorescence resonance energy transfer, biosensor
One of the reasons for the fable for hormones lies in the simple fact that it is easier to observe macroscopic changes, such as changes in the architecture of a plant than to determine metabolite levels, but also here new tools are urgently needed that allow quantification of these small molecules. Visualization of starch levels provided a significant advance, and in combination with mutant screens allowed to identify fundamental components of starch metabolism.7–9 The biggest advance for the signaling field was the development of advanced chemical and genetically encoded calcium dyes.10–12 No such dyes are available for hormones or metabolites, as soon as we try to determine levels of metabolites (or signaling molecules), we run into the issues of compartmentation and cellular differences in tissues. Today, the same enzymatic assays used decades ago are still widely used to determine metabolite levels. Although significant advances in chromatography and mass spectrometry based metabolite analysis have moved the study of metabolism to ‘omics’ era, compartmentalization of metabolism still presents a major challenge. Especially the large vacuoles of plant cells are a major obstacle, since even fractionation studies suffer from contamination. Moreover, with the current set of tools it is not possible to determine the dynamic changes in metabolite levels in different subcellular compartments in real time in vivo. Radiotracers have helped a lot to identify and quantify intermediates and to assemble pathways, originally using pulse labeling followed by paper chromatography. Today 13C-labeling is used together with mass spectrometry to obtain insights into metabolic flux control.13 This tool set for the first time enabled the comparison of mutants and study regulatory networks involved in sugar signaling. While significant, advances in radiotracer experiments do not provide cellular or subcellular information and only limited temporal resolution, they do provide efficient means for studying metabolite fluxes through complex and/or not well-defined pathways. Thus there is a clear need for metabolite specific dyes that can be targeted to subcellular compartments and that would enable flux measurements in response to environmental cues helping to push metabolic research back into the focus of signaling-related biology.
In 2002, we developed the first prototype “metabolic dye” FRET sensor for maltose.14,15 A similar glucose sensor was recently employed for measuring tracer-independent transport of glucose across the ER membrane of liver cells.16 After resolving some issues such as low signal-to-noise and gene silencing in plants, we are now able to compare glucose levels between cells in an intact root in real time.17 The parallel development of sucrose and phosphate sensors complements the set of tools, in future experiments providing a comparison of sucrose, phosphate and glucose fluxes in intact tissues with both temporal (below seconds) and spatial resolution (cellular and subcellular).18,19
The first experiments already led to a big surprise: glucose supplied to the root is rapidly taken up and is rapidly metabolized.17 Roots expressing the highest affinity sensor FLIPglu170n responded to glucose perfusion suggesting that the steady state glucose level in the root is less than 100 nM, the estimated detection limit for this sensor in these first experiments. The first experiments were limited by the mixing kinetics in the bath used for perfusion, while improvement of the chamber now allow for faster for glucose exchange. We estimate that glucose levels fall from a steady state level of approximately 5 mM in the cytosol when perfused with 5 mM glucose to below 100 nM in about three minutes. For the sensor with an affinity of 600 µM the rate of glucose accumulation, which is composed of the various rates that affect the steady state in the cytosol such as metabolism, compartmentation and transport across the plasma membrane, is in the range of 527 ± 77 µM glucose/min and that for glucose removal is 317 ± 37 (Fig. 1; Chaudhuri B, Frommer WB, unpublished). Questions that arise are: Which transport systems drive uptake? How much does the vacuole contribute to the observed flux and steady state levels? Is the capacity of hexokinase at levels below its Km still sufficient to phosphorylate glucose efficient enough to pull glucose below 100 nM or does hexokinase have different properties in vivo compared to what we know from the purified enzyme? Are there different transporters and enzymes contributing to flux in the low (1–10 mM) and the ultrahigh affinity (low µM) phases? Are there spatial differences in the root? Why do roots take up glucose so efficiently in the first place? The combination of the sensors with information from the expression-LEDs from Birnbaum and Benfey20 and specific knock-out mutants should help answering some of these questions.
Figure 1.
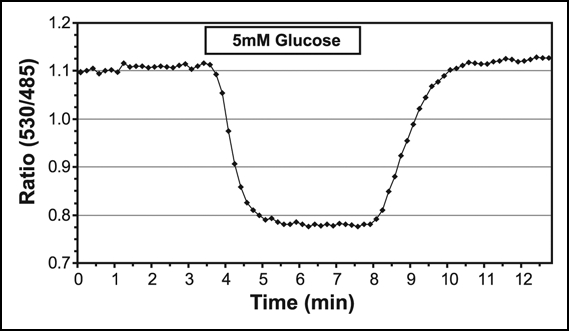
Quantitative analysis of glucose flux from an Arabidopsis root expressing FLIPglu-600µΔ13, a FRET sensor for glucose with an affinity of 600 µM. The root of a 10 day-old seedling was placed into a perfusion chamber and perfused with hydroponic medium with or without 5 mM glucose. eCFP was excited and emission was recorded for eCFP and eYFP every 10 seconds (essentially as decsribed in ref. 17). The emission intensities for a region-of-interest were averaged and the emission ratio was determined at the two wavelengths for each image of a time series and plotted on the Y-axis against time on the X-axis. Addition of glucose is indicated.
Another big surprise is the dramatic gradient of glucose across the plasma membrane, which has important implications for our understanding of transport processes across the plasma membrane as well as the intracellular membranes.17 Information about the gradients is relevant in the context of apo- and symplasmic unloading routes in roots21 and the contribution of proton-coupled transporters in cellular export.22 It will thus be interesting to follow the extracellular levels using surface-anchored sensors. Now that besides high sensitivity glucose FLIPs17 we also generated nanosensors for sucrose19 and phosphate,18 complementing the similar tool sets for calcium23 and pH,24 it is possible to compare multiple parameters and to follow flux at different levels and to calibrate against other influences.
The improvements of the signal-to-noise ratio of the FRET-based metabolite sensors25 makes the FLIPs a standard tool for every lab interested in measuring ion-, sugar- or amino acid flux in living cells. Since the nanosensors are genetically encoded, they can be used to characterize intracellular fluxes16,26 in any organism for which transformation protocols have been established. The existing sets of sensors are simple to use, constructs are available through Addgene and Arabidopsis lines from the Arabidopsis Stock Center. Detailed instructions for imaging can be found at: http://carnegiedpb.stanford.edu/research/frommer/research_frommer_protocols.php. These tools will hopefully become a standard system not only for physiological analyses, but in addition provide a new way for high throughput fluxomics studies.
Acknowledgements
This work was made possible by funds from the Department of Energy (DE-FG0204ER15542).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3643
References
- 1.Tengholm A, Teruel MN, Meyer T. Single cell imaging of PI3K activity and glucose transporter insertion into the plasma membrane by dual color evanescent wave microscopy. Science STKE. 2003;2003:PL4. doi: 10.1126/stke.2003.169.pl4. [DOI] [PubMed] [Google Scholar]
- 2.Leyser O. Dynamic integration of auxin transport and signalling. Curr Biol. 2006;16:R424–R433. doi: 10.1016/j.cub.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Wang ZY, Wang Q, Chong K, Wang F, Wang L, Bai M, Jia C. The brassinosteroid signal transduction pathway. Cell Res. 2006;16:427–434. doi: 10.1038/sj.cr.7310054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stitt M. Fructose 2,6-bisphosphate and plant carbohydrate metabolism. Plant Physiol. 1987;84:201–204. doi: 10.1104/pp.84.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holsbeeks I, Lagatie O, Van Nuland A, Van de Velde S, Thevelein JM. The eukaryotic plasma membrane as a nutrient-sensing device. Trends Biochem Sci. 2004;29:556–564. doi: 10.1016/j.tibs.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Santangelo GM. Glucose signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2006;70:253–282. doi: 10.1128/MMBR.70.1.253-282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu TS, Kofler H, Hausler RE, Hille D, Flugge UI, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, Steup M, Lue WL, Chen J, Weber A. The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell. 2001;13:1907–1918. doi: 10.1105/TPC.010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niittylä T, Comparot-Moss S, Lue WL, Messerli G, Trevisan M, Seymour MD, Gatehouse JA, Villadsen D, Smith SM, Chen J, Zeeman SC, Smith AM. Similar protein phosphatases control starch metabolism in plants and glycogen metabolism in mammals. J Biol Chem. 2006;281:11815–11818. doi: 10.1074/jbc.M600519200. [DOI] [PubMed] [Google Scholar]
- 9.Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC. A previously unknown maltose transporter essential for starch degradation in leaves. Science. 2004;303:87–89. doi: 10.1126/science.1091811. [DOI] [PubMed] [Google Scholar]
- 10.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 11.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 12.Romoser VA, Hinkle PM, Persechini A. Detection in living cells of Ca2+-dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence - A new class of fluorescent indicators. J Biol Chem. 1997;272:13270–13274. doi: 10.1074/jbc.272.20.13270. [DOI] [PubMed] [Google Scholar]
- 13.Raghevendran V, Gombert AK, Christensen B, Kotter P, Nielsen J. Phenotypic characterization of glucose repression mutants of Saccharomyces cerevisiae using experiments with 13C-labelled glucose. Yeast. 2004;21:769–779. doi: 10.1002/yea.1136. [DOI] [PubMed] [Google Scholar]
- 14.Fehr M, Frommer WB, Lalonde S. Visualization of maltose uptake in living yeast cells by fluorescent nanosensors. Proc Natl Acad Sci USA. 2002;99:9846–9851. doi: 10.1073/pnas.142089199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stitt M. Imaging of metabolites by using a fusion protein between a periplasmic binding protein and GFP derivatives: From a chimera to a view of reality. Proc Natl Acad Sci USA. 2002;99:9614–9616. doi: 10.1073/pnas.162375899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehr M, Takanaga H, Ehrhardt DW, Frommer WB. Evidence for high-capacity bidirectional glucose transport across the endoplasmic reticulum membrane by genetically encoded fluorescence resonance energy transfer nanosensors. Mol Cell Biol. 2005;25:11102–11112. doi: 10.1128/MCB.25.24.11102-11112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deuschle K, Chaudhuri B, Okumoto S, Lager I, Lalonde S, Frommer WB. Rapid metabolism of glucose detected with FRET glucose nanosensors in epidermal cells and intact roots of Arabidopsis RNA-silencing mutants. Plant Cell. 2006;18:2314–2325. doi: 10.1105/tpc.106.044073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu H, Lalonde S, Okumoto S, Looger LL, Scharff-Poulsen AM, Grossman AR, Kossmann J, Jakobsen I, Frommer WB. A novel analytical method for in vivo phosphate tracking. FEBS Lett. 2006;580:5885–5893. doi: 10.1016/j.febslet.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lager I, Looger LL, Hilpert M, Lalonde S, Frommer WB. Conversion of a putative Agrobacterium sugar-binding protein into a FRET sensor with high selectivity for sucrose. J Biol Chem. 2006;281:30875–30883. doi: 10.1074/jbc.M605257200. [DOI] [PubMed] [Google Scholar]
- 20.Birnbaum K, Jung JW, Wang JY, Lambert GM, Hirst JA, Galbraith DW, Benfey PN. Cell type-specific expression profiling in plants via cell sorting of protoplasts from fluorescent reporter lines. Nat Methods. 2005;2:615–619. doi: 10.1038/nmeth0805-615. [DOI] [PubMed] [Google Scholar]
- 21.Oparka KJ, Duckett CM, Prior DAM, Fisher DB. Real-time imaging of phloem unloading in the root tip of Arabidopsis. Plant J. 1994;6:759–766. [Google Scholar]
- 22.Carpaneto A, Geiger D, Bamberg E, Sauer N, Fromm J, Hedrich R. Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J Biol Chem. 2005;280:21437–21443. doi: 10.1074/jbc.M501785200. [DOI] [PubMed] [Google Scholar]
- 23.Mank M, Reiff DF, Heim N, Friedrich MW, Borst A, Griesbeck O. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophys J. 2006;90:1790–1796. doi: 10.1529/biophysj.105.073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulte A, Lorenzen I, Bottcher M, Plieth C. A novel fluorescent pH probe for expression in plants. Plant Methods. 2006;2:7. doi: 10.1186/1746-4811-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deuschle K, Okumoto S, Fehr M, Looger LL, Kozhukh L, Frommer WB. Construction and optimization of a family of genetically encoded metabolite sensors by semirational protein engineering. Protein Sci. 2005;14:2304–2314. doi: 10.1110/ps.051508105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehr M, Lalonde S, Ehrhardt DW, Frommer WB. Live imaging of glucose homeostasis in nuclei of COS-7 cells. J Fluoresc. 2004;14:603–609. doi: 10.1023/b:jofl.0000039347.94943.99. [DOI] [PubMed] [Google Scholar]


