Abstract
Rhizomatous axes of Nothia aphylla, a land plant from the 400-myr-old Rhynie chert, host a fungus that closely resembles Glomites rhyniensis (Glomeromycota), the endomycorrhizal fungus of the Rhynie chert plant Aglaophyton major. However, G. rhyniensis is an intercellular endophyte that becomes intracellular exclusively within a well-defined region of the cortex, while the fungus in N. aphylla initially is intracellular but later becomes intercellular in the cortex. We hypothesize that N. aphylla displays an alternative mode of colonization by endomycorrhizal fungi, perhaps related to the peculiar internal anatomy of the lower portion of the rhizomatous axis, in which the radial arrangement of cells, along with the virtual absence of intercellular spaces, provides no intercellular infection pathway into the cortex.
Key Words: Aglaophyton major, endomycorrhiza, Glomeromycota, Nothia aphylla, Early Devonian, Rhynie chert
The Early Devonian (c. 400 Ma) Rhynie chert is an in situ silicified hot springs environment that has become significant in our understanding of the complexity of life in early terrestrial ecosystems because of the extraordinary preservation of plants, animals, and microorganisms.1 Moreover, various associations and interactions between different organisms can be directly examined,2 including the earliest fossil examples of arbuscular endomycorrhizae.3,4 The Rhynie chert land plant Aglaophyton major is characterized by arching, stomatiferous prostrate axes that grow along the substrate surface, and form rhizoid-bearing bulges, usually around stomata, upon contact with the substrate. Extramatrical hyphae of the mycorrhizal fungal enter the axes through these stomata, and spread out through the intercellular system of the hypodermis and cortex, subsequently penetrating individual cells within a well-defined region of the cortex (i.e., the mycorrhizal arbuscule-zone) to form arbuscules.4
A recently published study5 reports on three fungal endophytes that (co-)occur in the Rhynie chert plant Nothia aphylla. This plant consists of upright aerial axes arising from a system of non-stomatiferous, subterranean rhizomatous axes characterized by a prominent ventral rhizoidal ridge.6,7 The rhizoidal ridge, which is unique among Rhynie chert land plants, consists of a rhizoid-bearing epidermis, a multi-layered hypodermis, files of parenchyma cells that connect to the stele, and extra-stelar conducting elements (Fig. 1A); intercellular spaces are virtually absent.
Figure 1.
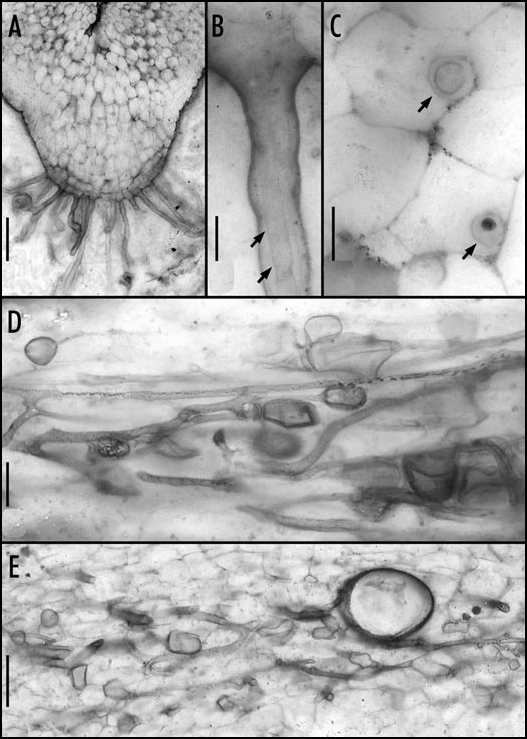
Nothia aphylla from the Lower Devonian Rhynie chert. (A) Ventral portion of a rhizomatous axis with rhizoidal ridge (cross section); bar = 250 µm. (B) Fungal hypha [arrows] entering the axis through a rhizoid; bar = 30 µm. (C) Sheathed intracellular hyphae [arrows] in hypodermal cells (transverse section); bar = 20 µm. (D) Intercellular hyphae and vesicles in the cortex (longitudinal section); bar scale = 50 µm. (E) Hyphae, vesicles and a thick-walled spore in the cortex (longitudinal section); bar = 100 µm. All images from the original paper; reproduced with permission.
One of the fungal endophytes in N. aphylla closely resembles Glomites rhyniensis (Glomeromycota), the endomycorrhizal fungus of A. major.4 In N. aphylla, this fungus occurs as an intracellular endophyte in rhizoids and tissues of the rhizoidal ridge. Moreover, it is abundant in the intercellular system of the cortex of both prostrate and proximal portions of aerial axes. The fungus enters the axes through rhizoids (Fig. 1B). Once in the hypodermis, hyphae become sheathed by cell wall material (Fig. 1C). In the cortex, the fungus produces intercellular vesicles (Fig. 1D) and thick-walled spores (Fig. 1E). Based on the presence of vesicles that are similar to those of G. rhyniensis, and spores like those in extant Glomeromycota, we hypothesize that this fungus is an endomycorrhizal member of the Glomeromycota; however, arbuscules have not been observed to date.
If this interpretation is accurate, N. aphylla displays an alternative pattern of colonization by endomycorrhizal fungi. Although the morphology of the fungus and distribution in N. aphylla correspond to that of G. rhyniensis in A. major, the infection pathway is distinctly different. While G. rhyniensis is an intercellular endophyte that penetrates individual cells exclusively within the mycorrhizal arbuscule-zone,4 the fungus of N. aphylla enters the plant as an intracellular endophyte, and remains intracellular until it reaches the cortex. The host plant apparently does not respond to the invading fungus because infected rhizoids are not altered morphologically. Once in the hypodermis, however, hyphae become separated from the host cell protoplast. This feature suggests a shift from (i) uncontrolled intracellular occurrence of the fungus in the rhizoids, to (ii) controlled intracellular occurrence in the rhizoidal ridge, to (iii) intercellular occurrence in the cortex.
The fact that the rhizomatous axes of N. aphylla are subterranean, along with the peculiar internal anatomy of the rhizoidal ridge, may have provided the selective pressure for an alternative mode of colonization by endomycorrhizal fungi. The fungus probably enters the plant through rhizoids because the axes are non-stomatiferous. Moreover, the morphology and radial arrangement of cells in the rhizoidal ridge, along with the virtual absence of intercellular spaces, perhaps does not provide an intercellular infection pathway into the cortex. We speculate that N. aphylla tolerated intracellular penetration in the rhizoids and within the tissues of the rhizoidal ridge in order to become inoculated. Tolerating (or even facilitating) intracellular penetration within a limited area of the axis may simultaneously have provided the plant with a means of recognizing and subsequently distinguishing the endomycorrhizal fungus from potentially harmful parasites (e.g., by surface features of the hyphae or chemical signals). Once recognized, the endomycorrhizal fungi become sheathed and “guided” through the ridge without being able to extract nutrients from the host, and into the cortex where intracellular penetration is not longer possible. The parasites, once recognized, are confined in the tissues of the rhizoidal ridge by specific or unspecific host responses, e.g., secondarily thickened cell walls.5 Conversely, if the endomycorrhizal fungus entered the plant through surface openings, and spread out exclusively through the intercellular system, the mechanisms that might confine simultaneous parasite infections were probably much more limited.
Endomycorrhizal relationships are believed to have evolved from parasitic interactions.8 It has been postulated that modern enodomycorrhizal fungi in some way control parasites because both compete for the same resources.9 It may be that, during the evolution of fungal endophytism, the initial benefits of mycorrhizae included protection of the host from pathogenic fungi.10 Nothia aphylla from the Lower Devonian Rhynie chert adds support to this hypothesis, and may demonstrate that more than a single pattern of colonization by endomycorrhizal fungi occurred during the early evolution of land plants.
Acknowledgements
This study was supported by funds from the National Science Foundation (EAR-0542170), the Alexander von Humboldt-Foundation (V-3.FLF-DEU/1064359), and the Deutsche Forschungsgemeinschaft (Ke 584/13-1).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3970
References
- 1.Kerp H, Hass H. De onder-devonische Rhynie chert — Het oudste en meest compleet bewaard gebleven terrestrische ecosysteem. Grondbor en Hamer. 2004;58:33–50. [Google Scholar]
- 2.Taylor TN, Krings M. Fossil microorganisms and land plants: Associations and interactions. Symbiosis. 2005;40:119–135. [Google Scholar]
- 3.Remy W, Taylor TN, Hass H, Kerp H. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc Natl Acad Sci USA. 1994;91:11841–11843. doi: 10.1073/pnas.91.25.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor TN, Remy W, Hass H, Kerp H. Fossil arbuscular mycorrhizae from the Early Devonian. Mycologia. 1995;87:560–573. [Google Scholar]
- 5.Krings M, Taylor TN, Hass H, Kerp H, Dotzler N, Hermsen EJ. Fungal endophytes in a 400-million-yr-old land plant: Infection pathways, spatial distribution, and host responses. New Phytol. 2007 doi: 10.1111/j.1469-8137.2007.02008.x. (DOI 10.1111/j.1469-8137.2007.02008.x) [DOI] [PubMed] [Google Scholar]
- 6.Kerp H, Hass H, Mosbrugger V. New data on Nothia aphylla Lyon 1964 ex El-Saadawy et Lacey 1979, a poorly known plant from the Lower Devonian Rhynie chert. In: Gensel PG, Edwards D, editors. Plants invade the land - Evolutionary and environmental perspectives. New York: Columbia Univ Press; 2001. pp. 52–82. [Google Scholar]
- 7.Daviero-Gomez V, Kerp H, Hass H. Nothia aphylla: The issue of clonal development in early land plants. Int J Plant Sci. 2005;166:319–326. [Google Scholar]
- 8.Parniske M. Intracellular accommodation of microbes by plants: A common developmental program for symbiosis and disease? Curr Opin Plant Biol. 2000;3:320–328. doi: 10.1016/s1369-5266(00)00088-1. [DOI] [PubMed] [Google Scholar]
- 9.Graham JH. What do root pathogens see in mycorrhizas? New Phytol. 2001;149:357–359. doi: 10.1046/j.1469-8137.2001.00077.x. [DOI] [PubMed] [Google Scholar]
- 10.Brundrett MC. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002;154:275–304. doi: 10.1046/j.1469-8137.2002.00397.x. [DOI] [PubMed] [Google Scholar]


