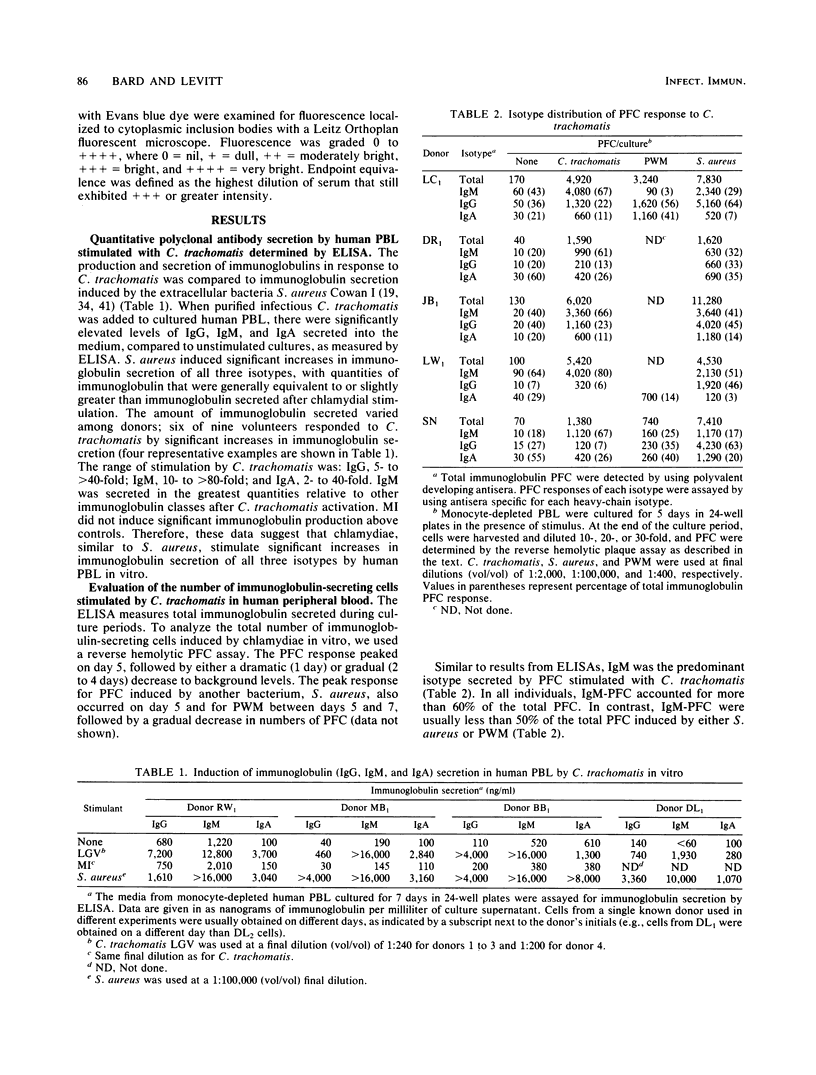
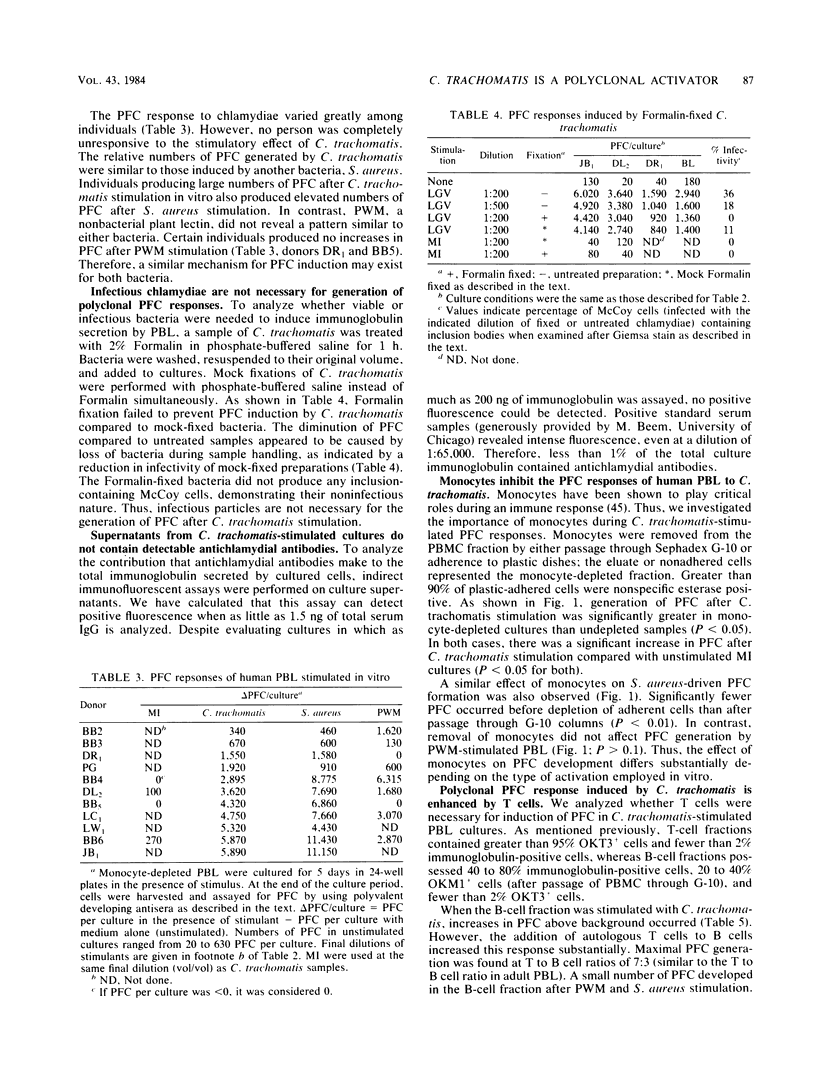
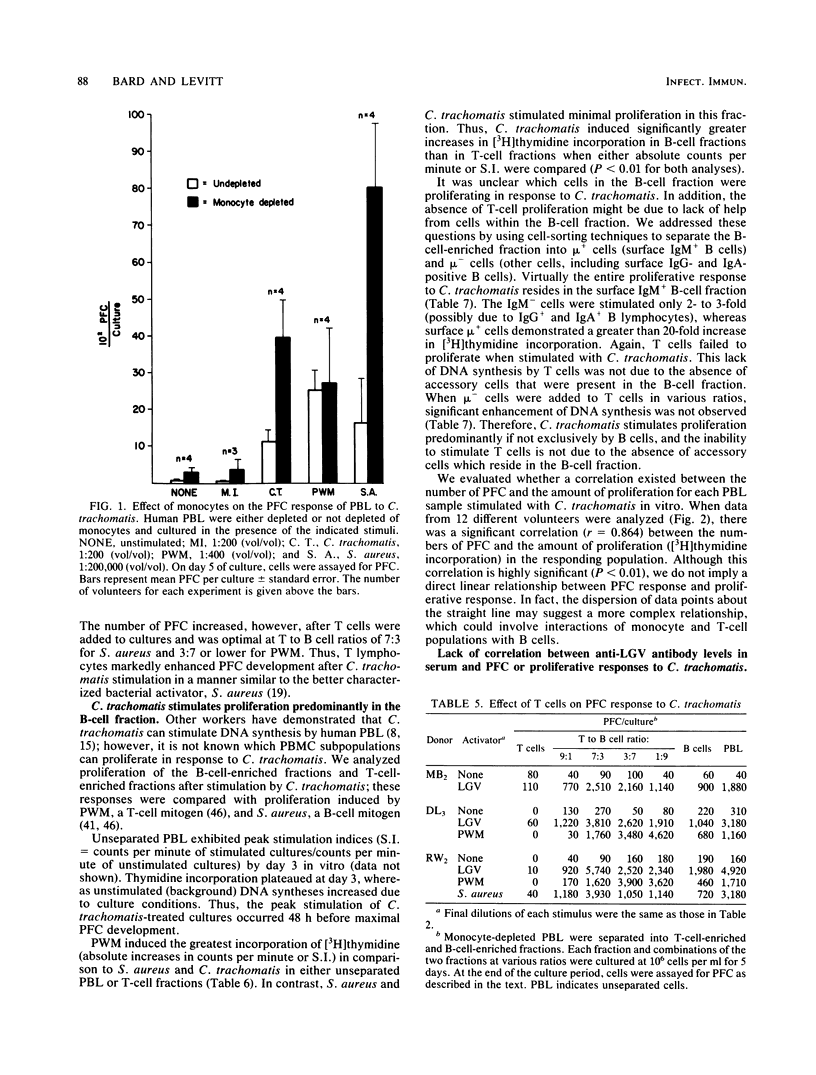
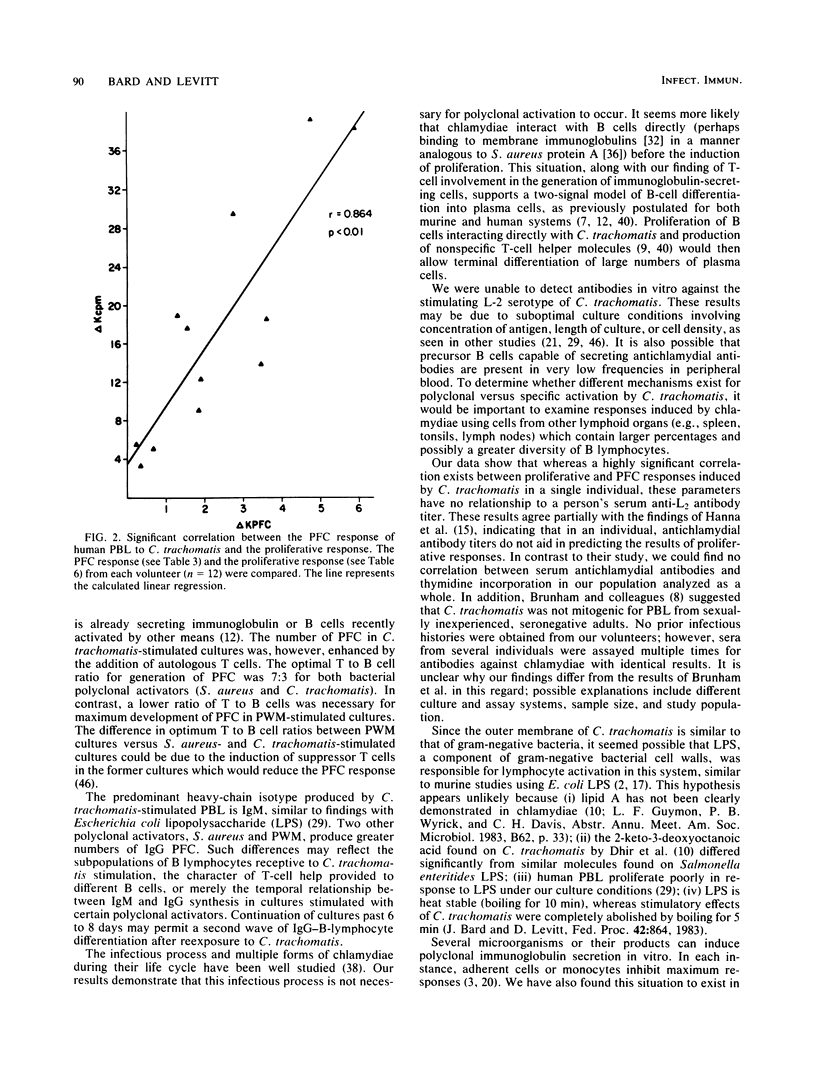
Abstract
Infectious Chlamydia trachomatis (LGV strain), obligate intracellular bacteria, stimulated human peripheral blood lymphocytes to proliferate and secrete immunoglobulins in vitro. In contrast, mock-infected preparations were unable to induce similar responses in peripheral blood lymphocytes. Although levels of immunoglobulin secreted into the media of LGV-stimulated cultures were greater than 10 micrograms/ml, we estimated that less than 1% of these molecules were directed against the bacteria itself, suggesting polyclonal antibody production. Since stimulation with Formalin-killed bacteria resulted in comparable numbers of plaque-forming cells (PFC) as infectious particles, we concluded that the polyclonal immunoglobulin response was not dependent on the in vitro chlamydial infectious process. The polyclonal PFC response induced by LGV was highly sensitive to monocyte inhibition. Although LGV induced proliferation of predominantly B cells, the numbers of generated PFC was increased by the addition of autologous T cells. Neither lymphocyte proliferation nor PFC responses of normal human volunteers correlated significantly with the presence or titer of antichlamydial antibodies in their sera.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Melchers F. Maturation of mitogen-activated bone marrow-derived lymphocytes in the absence of proliferation. Eur J Immunol. 1974 Aug;4(8):533–539. doi: 10.1002/eji.1830040803. [DOI] [PubMed] [Google Scholar]
- Andersson J., Sjöberg O., Möller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur J Immunol. 1972 Aug;2(4):349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- Banck G., Forsgren A. Many bacterial species are mitogenic for human blood B lymphocytes. Scand J Immunol. 1978;8(4):347–354. doi: 10.1111/j.1365-3083.1978.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Beem M. O., Saxon E. M. Respiratory-tract colonization and a distinctive pneumonia syndrome in infants infected with Chlamydia trachomatis. N Engl J Med. 1977 Feb 10;296(6):306–310. doi: 10.1056/NEJM197702102960604. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bretscher P. A. The two signal model for b cell induction. Transplant Rev. 1975;23:37–48. doi: 10.1111/j.1600-065x.1975.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Martin D. H., Kuo C. C., Wang S. P., Stevens C. E., Hubbard T., Holmes K. K. Cellular immune response during uncomplicated genital infection with Chlamydia trachomatis in humans. Infect Immun. 1981 Oct;34(1):98–104. doi: 10.1128/iai.34.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- Chiorazzi N., Fu S. M., Kunkel H. G. Induction of polyclonal antibody synthesis by human allogeneic and autologous helper factors. J Exp Med. 1979 Jun 1;149(6):1543–1548. doi: 10.1084/jem.149.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir S. P., Hakomori S., Kenny G. E., Grayston J. T. Immunochemical studies on chlamydial group antigen (presence of a 2-keto-3-deoxycarbohydrate as immunodominant group). J Immunol. 1972 Jul;109(1):116–122. [PubMed] [Google Scholar]
- Dutton R. W., Mishell R. I. Cell populations and cell proliferation in the in vitro response of normal mouse spleen to heterologous erythrocytes. Analysis by the hot pulse technique. J Exp Med. 1967 Sep 1;126(3):443–454. doi: 10.1084/jem.126.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkoff R. J., Zhu L. P., Fauci A. S. Separate signals for human B cell proliferation and differentiation in response to Staphylococcus aureus: evidence for a two-signal model of B cell activation. J Immunol. 1982 Jul;129(1):97–102. [PubMed] [Google Scholar]
- Gathings W. E., Lawton A. R., Cooper M. D. Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977 Nov;7(11):804–810. doi: 10.1002/eji.1830071112. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hanna L., Schmidt L., Sharp M., Stites D. P., Jawetz E. Human cell-mediated immune responses to chlamydial antigens. Infect Immun. 1979 Feb;23(2):412–417. doi: 10.1128/iai.23.2.412-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D. M. Synergy between T cell-replacing factor and bacterial lipopolysaccharides (LPS) in the primary antibody response in vitro: a model for lipopolysaccharide adjuvant action. J Immunol. 1979 Apr;122(4):1421–1426. [PubMed] [Google Scholar]
- Jerrells T. R., Dean J. H., Richardson G. L., Herberman R. B. Depletion of monocytes from human peripheral blood mononuclear leukocytes: comparison of the sephadex G-10 column method with other commonly used techniques. J Immunol Methods. 1980;32(1):11–29. doi: 10.1016/0022-1759(80)90113-1. [DOI] [PubMed] [Google Scholar]
- Kim Y. T., Schwartz A., Blum J., Weksler M. E. The plaque-forming cell response of human blood lymphocytes. I. PFC response of lymphocytes to formalin-treated staphylocci. Cell Immunol. 1979 Dec;48(2):308–317. doi: 10.1016/0008-8749(79)90125-4. [DOI] [PubMed] [Google Scholar]
- Knapp W., Baumgartner G. Monocyte-mediated suppression of human B lymphocyte differentiation in vitro. J Immunol. 1978 Sep;121(3):1177–1183. [PubMed] [Google Scholar]
- Kunori T., Ringdén O., Möller E. Optimal conditions for polyclonal antibody secretion and DNA synthesis in human blood and spleen lymphocytes by lipopolysaccharide. Scand J Immunol. 1978;8(5):451–458. doi: 10.1111/j.1365-3083.1978.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Kuo C. C. Cultures of Chlamydia trachomatis in mouse peritoneal macrophages: factors affecting organism growth. Infect Immun. 1978 May;20(2):439–445. doi: 10.1128/iai.20.2.439-445.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Chen W. J. A mouse model of Chlamydia trachomatis pneumonitis. J Infect Dis. 1980 Feb;141(2):198–202. doi: 10.1093/infdis/141.2.198. [DOI] [PubMed] [Google Scholar]
- Lassus A., Mustakallio K. K., Wager O. Auto-immune serum factors and IgA elevation in lymphogranuloma venereum. Ann Clin Res. 1970 Mar;2(1):51–56. [PubMed] [Google Scholar]
- Lee C. K. Interaction between a trachoma strain of Chlamydia trachomatis and mouse fibroblasts (McCoy cells) in the absence of centrifugation. Infect Immun. 1981 Feb;31(2):584–591. doi: 10.1128/iai.31.2.584-591.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D., Dagg M. K. Human B-lymphocyte subpopulations. II. Plasma cell differentiation of isotype-specific B lymphocytes from peripheral blood. Clin Immunol Immunopathol. 1981 Oct;21(1):50–61. doi: 10.1016/0090-1229(81)90194-x. [DOI] [PubMed] [Google Scholar]
- Levitt D., Duber-Stull D., Lawton A. R. T-cell-dependent and independent plasma cell differentiation induced by Escherichia coli Lipopolysaccharide in human peripheral blood lymphocytes. Clin Immunol Immunopathol. 1981 Mar;18(3):309–321. doi: 10.1016/0090-1229(81)90124-0. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., Batteiger B., Jones R. B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982 Dec;38(3):1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Ripa K. T., Mårdh P. A. Cultivation of Chlamydia trachomatis in cycloheximide-treated mccoy cells. J Clin Microbiol. 1977 Oct;6(4):328–331. doi: 10.1128/jcm.6.4.328-331.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., del Prete G., Maggi E., Biagiotti R., Almerigogna F., Ricci M. Demonstration on protein A of two distinct immunoglobulin-binding sites and their role in the mitogenic activity of Staphylococcus aureus Cowan I on human B cells. J Immunol. 1982 Aug;129(2):596–602. [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. A third signal in B cell activation given by TRF. Transplant Rev. 1975;23:176–188. doi: 10.1111/j.1600-065x.1975.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Schuurman R. K., Gelfand E. W., Dosch H. M. Polyclonal activation of human lymphocytes in vitro. I. Characterization of the lymphocyte response to a T cell-independent B cell mitogen. J Immunol. 1980 Aug;125(2):820–826. [PubMed] [Google Scholar]
- Senyk G., Kerlan R., Stites D. P., Schanzlin D. J., Ostler H. B., Hanna L., Keshishyan H., Jawetz E. Cell-mediated and humoral immune responses to chlamydial antigens in guinea pigs infected ocularly with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1981 Apr;32(1):304–310. doi: 10.1128/iai.32.1.304-310.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipple M. A., Beem M. O., Saxon E. M. Clinical characteristics of the afebrile pneumonia associated with Chlamydia trachomatis infection in infants less than 6 months of age. Pediatrics. 1979 Feb;63(2):192–197. [PubMed] [Google Scholar]
- Unanue E. R. The regulation of lymphocyte functions by the macrophage. Immunol Rev. 1978;40:227–255. doi: 10.1111/j.1600-065x.1978.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Polyclonal B-cell activators in the study of the regulation of immunoglobulin synthesis in the human system. Adv Immunol. 1982;32:1–63. doi: 10.1016/s0065-2776(08)60720-8. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Grubbs B., Sumaya C. V. The role of antibody in host defense against the agent of mouse pneumonitis. J Infect Dis. 1982 Feb;145(2):200–205. doi: 10.1093/infdis/145.2.200. [DOI] [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A., Ivins B. E. Interaction of Chlamydia psittaci with mouse peritoneal macrophages. Infect Immun. 1978 Mar;19(3):1061–1067. doi: 10.1128/iai.19.3.1061-1067.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong E. C., Klebanoff S. J., Kuo C. C. Toxic effect of human polymorphonuclear leukocytes on Chlamydia trachomatis. Infect Immun. 1982 Aug;37(2):422–426. doi: 10.1128/iai.37.2.422-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



