Abstract
We have demonstrated previously in insulin-sensitive skeletal muscle that lithium, an alkali metal and non-selective inhibitor of glycogen synthase kinase-3 (GSK-3), activates glucose transport by engaging the stress-activated p38 mitogen-activated protein kinase (p38 MAPK). However, it is presently unknown whether this same mechanism underlies lithium action on the glucose transport system in insulin-resistant skeletal muscle. We therefore assessed the effects of lithium on basal and insulin-stimulated glucose transport, glycogen synthesis, insulin signalling (insulin receptor (IR), Akt, and GSK-3), and p38 MAPK in soleus muscle from female obese Zucker rats. Lithium (10 mM LiCl) increased basal glucose transport by 49% (p < 0.05) and net glycogen synthesis by 2.4-fold (p < 0.05). In the absence of insulin, lithium did not induce IR tyrosine phosphorylation, but did enhance (p < 0.05) Akt ser473 phosphorylation (40%) and GSK-3ß ser9 phosphorylation (88%). Lithium potentiated (p < 0.05) the stimulatory effects of insulin on glucose transport (74%), glycogen synthesis (2.4-fold), Akt ser473 phosphorylation (39%), and GSK-3ß ser9 phosphorylation (36%), and elicited robust increases (p < 0.05) in p38 MAPK phosphorylation both in the absence (100%) or presence (88%) of insulin. The selective p38 MAPK inhibitor A304000 (10 μM) completely blocked basal activation of glucose transport by lithium, and significantly reduced (42%, p < 0.05) the lithium-induced enhancement of insulin-stimulated glucose transport in insulin-resistant muscle. These results indicate that lithium enhances both basal and insulin-stimulated glucose transport and glycogen synthesis in insulin-resistant skeletal muscle of female obese Zucker rats, and that these lithium-dependent effects are associated with enhanced Akt and GSK-3ß serine phosphorylation. As in insulin-sensitive muscle, the lithium-induced activation of glucose transport in insulin-resistant skeletal muscle is dependent on the engagement of p38 MAPK.
Keywords: Obese Zucker rat, Akt, glycogen synthase kinase-3, A304000
Introduction
Insulin action on the glucose transport process in skeletal muscle involves the sequential engagement of several intracellular signalling factors (reviewed in Shepherd & Kahn, 1999; Zierath et al., 2000; Henriksen & Dokken, 2006). Insulin initially binds and activates the insulin receptor (IR), which in turn mediates tyrosine phosphorylation of IR substrates (IRS, primarily IRS-1 and IRS-2 in muscle), allowing for engagement of phosphatidylinositol-3-kinase and eventually the serine/threonine kinase Akt. This series of events elicits the translocation of GLUT-4 glucose transporters to the sarcolemmal membrane, where they facilitate the glucose transport process. Activated Akt also mediates serine phosphorylation of glycogen synthase kinase-3 (GSK-3) (Cross et al., 1995), thereby inhibiting GSK-3 and contributing to glycogen synthesis by removing the inhibitory action of this enzyme on glycogen synthase (Parker et al., 1983; Roach 1990; Zhang et al., 1993).
Evidence in the literature also supports a role of p38 mitogen-activated protein kinase (p38 MAPK) in the regulation of the glucose transport system in insulin-sensitive skeletal muscle (Somwar et al., 2000; Kim et al., 2006; Harrell et al., 2007), potentially involving enhanced GLUT-4 activity (Sweeney et al., 1999; Somwar et al., 2000; Konrad et al., 2001; Somwar et al., 2002). However, this concept is not universally accepted, and evidence against a specific role of p38 MAPK in the regulation of insulin-stimulated glucose transport activity in cultured myocytes has been reported (Antonescu et al., 2005).
Insulin resistance of the skeletal muscle glucose transport system is associated with the development of type 2 diabetes and several cardiovascular risk factors (reviewed in Zierath et al., 2000; Henriksen & Dokken, 2006). The multifactorial etiology of skeletal muscle insulin resistance has been investigated at the molecular level in the obese Zucker rat, a model of pre-diabetes (Mathe, 1995; Henriksen & Dokken, 2006). Defective insulin-stimulated glucose transport activity in skeletal muscle of the obese Zucker rat (Crettaz et al., 1980; Henriksen & Jacob, 1995; Etgen et al., 1996) can be attributed to reduced GLUT-4 translocation (King et al., 1993, Etgen et al., 1996) arising from impairments in the insulin signalling cascade, including decreased tyrosine phosphorylation of IR (Saengsirusuwan et al., 2004) and IRS-1 (Anai et al., 1998; Saengsirisuwan et al., 2004), reduced Akt activation (Anai et al., 1998; Saengsirisuwan et al., 2004), and GSK-3 over-activity (Henriksen and Teachey, 2007). Pharmaceutical interventions to improve insulin action in skeletal muscle, such as GSK-3 inhibitors (Eldar-Finkelman & Ilouz, 2003; Wagman et al., 2004; Henriksen & Dokken, 2006), are designed to specifically target defects in insulin signalling.
The alkali metal lithium, a non-selective GSK-3 inhibitor (Henriksen & Dokken, 2006), has been utilized to investigate the regulation of the glucose transport system in mammalian skeletal muscle. In insulin-sensitive skeletal muscle, lithium cations enhance glucose transport in the absence or presence of insulin (Tabata et al., 1994; Fürnsinn et al., 1997; Henriksen et al., 2003; Harrell et al., 2007), actions associated with augmented serine phosphorylation of GSK-3ß (indicative of GSK-3 inhibition) and dependent on increased p38 MAPK activation (Harrell et al., 2007). Limited information is available regarding the effect of lithium on glucose utilization in insulin-resistant skeletal muscle. Lithium increases glucose transport activity in isolated muscle of the pre-diabetic obese Zucker rat (Fürnsinn et al., 1997) and the type 2 diabetic Zucker Diabetic Fatty rat (Henriksen et al., 2003) and enhances glycogen synthesis and glycogen synthase activity in isolated skeletal muscle of these rodent models of insulin resistance (Fürnsinn et al., 1997; Henriksen et al., 2003). However, the potential underlying molecular mechanisms for these actions of lithium in insulin-resistant skeletal muscle have not yet been identified.
In the context of the foregoing information, the present investigation was designed to address two primary aims:(1) to examine whether lithium activates glucose transport activity in isolated insulin-resistant skeletal muscle of the female obese Zucker rat by engaging critical elements of the insulin signalling cascade, including including IR tyrosine phosphorylation, Akt ser473 phosphorylation, and GSK-3β ser9 phosphorylation; and (2) to assess the role of p38 MAPK activation in the action of lithium on the glucose transport system in insulin-resistant rat skeletal muscle.
Materials and methods
Animals and muscle preparation
Female obese (fa/fa) Zucker rats were purchased from Harlan (Indianapolis, IN) and were used at 9-11 weeks of age and with body weights in the range of 300-350 g. Animals were housed in a temperature-controlled room (20-22°C) with a 12:12 hour light:dark cycle (lights on from 7 a.m. to 7 p.m.) at the Central Animal Facility of the University of Arizona. The animals had free access to chow (Teklad 7001 mouse/rat diet; Madison, WI) and water. All procedures were approved by the University of Arizona Institutional Animal care and Use Committee. Before each experiment, animals were restricted to 4 g of chow overnight starting at 5 p.m. Experiments began at 8 a.m. the next morning. Animals were deeply anaesthetized using an intraperitoneal injection of pentobarbital sodium (50 mg/kg body wt) and both soleus muscles were removed and prepared in strips weighing ~25-35 mg (Henriksen et al., 1990).
Muscle incubations
Muscles were incubated for 1 h at 37°C in oxygenated Krebs-Henseleit buffer containing 8 mM glucose, 32 mM mannitol, and 0.1% BSA (radio-immunoassay grade, Sigma, St. Louis, MO), in the absence or presence of 10 mM LiCl without or with 5 mU/ml insulin (Humulin R, Eli Lilly, Indianapolis, IN). In some experiments, muscles were incubated with A304000 (10 μM), a highly selective inhibitor of the p38 MAPK (Somwar et al., 2002; Kim et al., 2006) (kindly provided by Abbott Laboratories, Abbott Park, IL).
Determination of glucose transport activity and glycogen synthesis
At the end of the 1 h initial incubation, in vitro glucose transport activity was assessed as described previously (Henriksen & Jacob, 1995) using 1 mM 2-deoxy-[1,2-3H] glucose (2-DG) (300 μCi/mmol) and 39 mM [U-14C] mannitol (0.8 μCi/mmol) (both from Sigma). After a 20-min exposure to the radio-labelled 2-DG and mannitol, muscles were removed, trimmed of excess fat and connective tissue, quickly frozen between aluminium blocks cooled in liquid nitrogen, and weighed and dissolved in 0.5 ml 0.5 N NaOH. Five ml of scintillant were added, and the specific intracellular accumulation of 2-DG was determined as described previously (Henriksen & Jacob, 1995). This method for assessing glucose transport activity in isolated muscle has been validated (Hansen et al., 1994). In addition, in some experiments, the incorporation of 14C-glucose (10 μCi/ml) in the presence of 5 mM glucose, an index of net glycogen synthesis, was determined as described previously (Henriksen et al., 1986).
Measurement of signalling factors
Analysis of tyrosine-phosphorylated IR-β was performed via immunoprecipitation and immunoblotting. Muscles were homogenized in 50 mM HEPES (pH 7.4) containing 150 mM NaCL, 1% Triton X-100, 2 μg/ml aprotinin, 0.2 mM leupeptin, 2 mM PMSF, 0.1 mM antipain, and 0.5 units/ml a-macroglobulin. After a 20-min incubation on ice, the homogenates were centrifuged at 13,000 g for 20 min at 4°C. A portion of the lysates was analysed for total protein concentration using the bicinchoninic acid (BCA) method (Sigma). The lysate samples were then diluted to equal total protein concentrations. For analysis of phosphorylated IR-β, 1 ml of diluted homogenate was immunoprecipitated with 15 μl of recombinant agarose-conjugated anti-phosphotyrosine antibody (4G10, Upstate Biotechnology, Lake Placid, NY) and gently rocked overnight at 4°C. The solutions were centrifuged for approximately 5 sec. The supernatants were removed, and the beads were washed three times with PBS and then boiled for 5 min in SDS-PAGE sample buffer. Samples were separated on either 7.5% polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA) and blotted electrophoretically onto nitrocellulose paper. Immunoprecipitates were also immunoblotted with an antibody against IR β-subunit (Santa Cruz Biotechnology, Santa Cruz, CA).
Analyses of phosphorylated Akt, GSK-3, p38 MAPK, AMP-dependent protein kinase (AMPK), and c-jun N-terminal kinase (JNK) were performed via immunoblotting. Muscles were homogenized in 50 mM HEPES (pH 7.4) containing 150 mM NaCl, 20 mM Na pyrophosphate, 20 mM β-glycerophosphate, 10 mM NaF, 2 mM Na3VO4, 2 mM EDTA (pH 8.0), 1% Triton X-100, 10% glycerol, 1 mM MgCl2, 1 mM CaCl2, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 2 mM PMSF. After a 20-min incubation on ice, the homogenates were centrifuged at 13,000 g for 20 min at 4°C. A portion of the lysates were analysed for total protein concentration. The remainder of the lysates was used for Western blot analysis. After diluting the samples to an equal total protein concentration and denaturing by boiling for 5 min with SDS-PAGE sample buffer, samples were separated on 7.5% or 12% polyacrylamide gels (Bio-Rad) and blotted electrophoretically onto nitrocellulose paper. For evaluation of phosphorylated Akt, GSK-3, p38 MAPK, AMPK, and JNK, blots were incubated with commercially available antibodies (Cell Signalling, Technology, Beverly, MA) against phospho-Akt (ser473), phospho-GSK-3 α/β (ser21/ser9), phospho-p38 MAPK (thr180/tyr182), phospho-AMPKα (thr172), and phospho-JNK1/2 (thr183/tyr185), or with antibodies against total Akt1/2 (Cell Signalling), GSK-3 α/β (Upstate), p38 MAPK (Santa Cruz), AMPKα (Cell Signalling), and JNK1/2 (Cell Signalling). In our hands, we can only detect phosphorylation of the β-isoform (at ser9) of GSK-3 (Dokken et al., 2005; Sloniger et al., 2005). After incubation with horseradish peroxidase-conjugated secondary antibodies, proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) using the ChemiDoc system (Bio-Rad), and band density was quantified using Quantity One software (Bio-Rad).
Statistical analysis
Data are expressed as means ± SE. Paired Student's t-tests were employed to determine statistically significant differences in group means when soleus splits derived from the same muscle were used to assess the specific effects of lithium (Figures 1-6) or an inhibitor (A304000; Figures 7 and 8). A level of p < 0.05 was set for statistical significance.
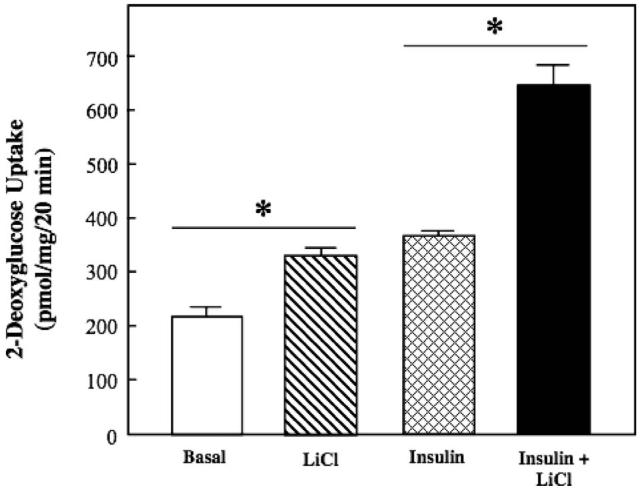
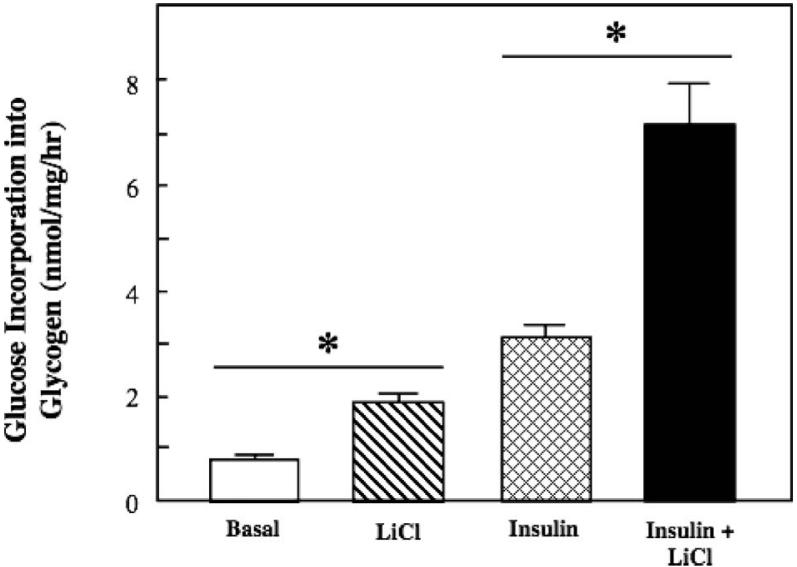
Figure 1.
Effect of lithium on basal and insulin-stimulated glucose transport activity in insulin-resistant skeletal muscle. Soleus muscle from obese Zucker rats was incubated in the absence or presence of 10 mM LiCl without or with 5 mU/ml insulin, and 2-deoxyglucose uptake was assessed as described in Materials and methods. Values are means±SE for 4-5 muscles per group. *p < 0.05, LiCl vs. basal or insulin + LiCl vs. insulin, by paired Student's t-test.
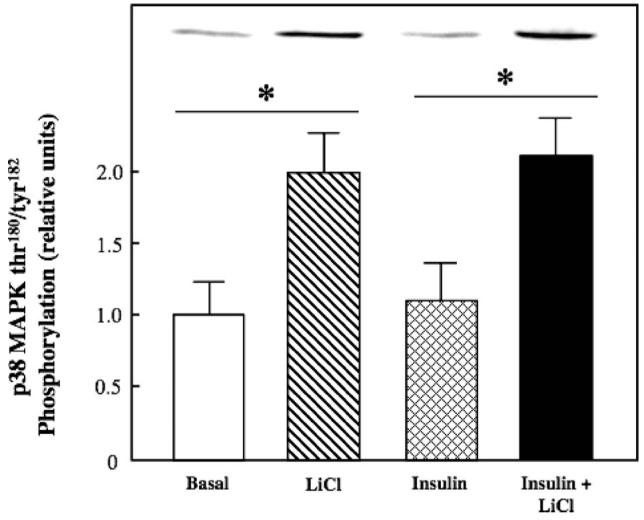
Figure 6.
Effect of lithium on basal and insulin-stimulated p38 MAPK phosphorylation in insulin-resistant skeletal muscle. Soleus muscles from obese Zucker rats was incubated in the absence or presence of 10 mM LiCl without or with 5 mU/ml insulin, and thr180/tyr182 phosphorylation on p38 MAPK was assessed as described in Materials and methods. Representative bands from the autoradiograph are displayed at the top of the figure. Values are means±SE for 4-5 muscles per group. *p < 0.05, LiCl vs. basal or insulin + LiCl vs. insulin, by paired Student's t-test.
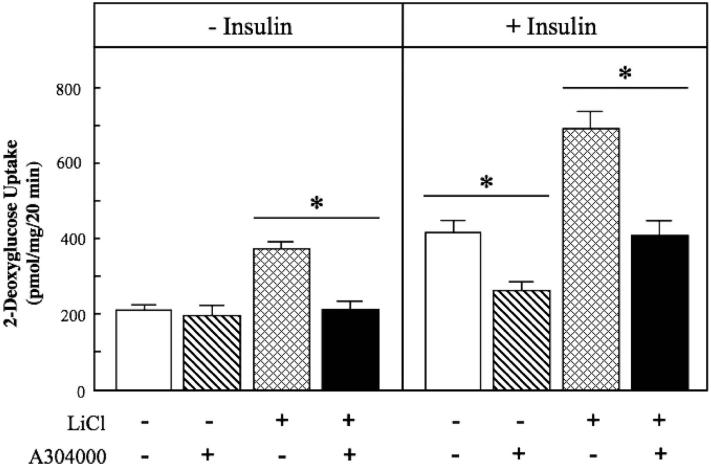
Figure 7.
Effect of p38 MAPK inhibition on activation of glucose transport activity by lithium and insulin in insulin-resistant skeletal muscle. Soleus muscle from obese Zucker rats was incubated in the absence or presence of 10 mM LiCl or in the absence or presence of 5 mU/ml insulin, without or with the p38 MAPK inhibitor A304000 (10 μM). Values are means±SE for 4-5 muscles per group. *p < 0.05, vs. the same treatment in the absence of A304000, by paired Student's t-test.
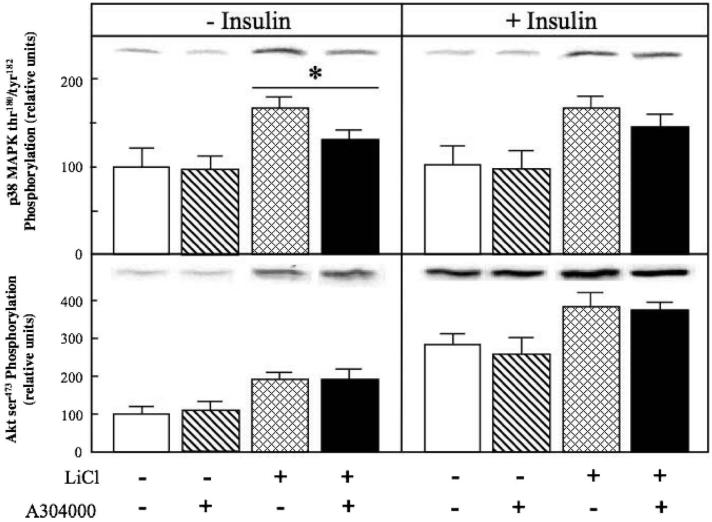
Figure 8.
Effect of p38 MAPK inhibition on the effects of lithium and insulin on p38 MAPK phosphorylation and Akt phosphorylation in insulin-resistant skeletal muscle. Soleus muscle from obese Zucker rats was incubated in the absence or presence of 10 mM LiCl or in the absence or presence of 5 mU/ml insulin, without or with the p38 MAPK inhibitor A304000 (10 μM). Muscles were then assayed for p38 MAPK thr180/tyr182 phosphorylation and Akt ser473 phosphorylation as described in Materials and methods. Values are means±SE for 4 muscles per group. *p < 0.05, vs. the same treatment in the absence of A304000, by paired Student's t-test.
Results
Lithium action on glucose transport and glycogen synthesis in insulin-resistant muscle
Lithium increased basal glucose transport activity in isolated soleus muscle of the insulin-resistant obese Zucker rat by 49% (p < 0.05) (Figure 1). Insulin by itself enhanced glucose transport activity by 72%. Importantly, this effect of insulin on glucose transport activity in the insulin-resistant skeletal muscle was significantly potentiated by lithium (74%, p < 0.05), to a greater extent than can be accounted for by the individual additive effects of lithium and insulin.
Glycogen synthesis in insulin-resistant skeletal muscle was similarly modulated by lithium (Figure 2). Lithium stimulated basal glucose incorporation into glycogen by 2.4-fold (p < 0.05). Insulin increased glycogen synthesis in the insulin-resistant soleus by almost 4-fold. Lithium and insulin in combination further increased insulin-stimulated glycogen synthesis by 2.4-fold (p < 0.05), again much more than the theoretical additive effects of the individual compounds.
Figure 2.
Effect of lithium on basal and insulin-stimulated glycogen synthesis in insulin-resistant skeletal muscle. Soleus muscle from obese Zucker rats was incubated in the absence or presence of 10 mM LiCl without or with 5 mU/ml insulin, and glucose incorporation into glycogen was assessed as described in Materials and methods. Values are means±SE for 5 muscles per group. *p < 0.05, LiCl vs. basal or insulin + LiCl vs. insulin, by paired Student's t-test.
Lithium action on insulin signalling in insulin-resistant muscle
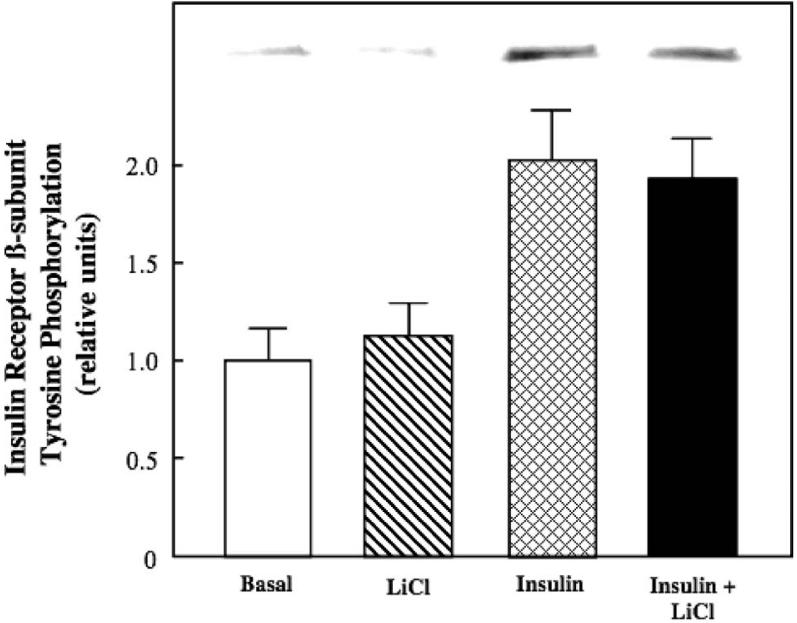
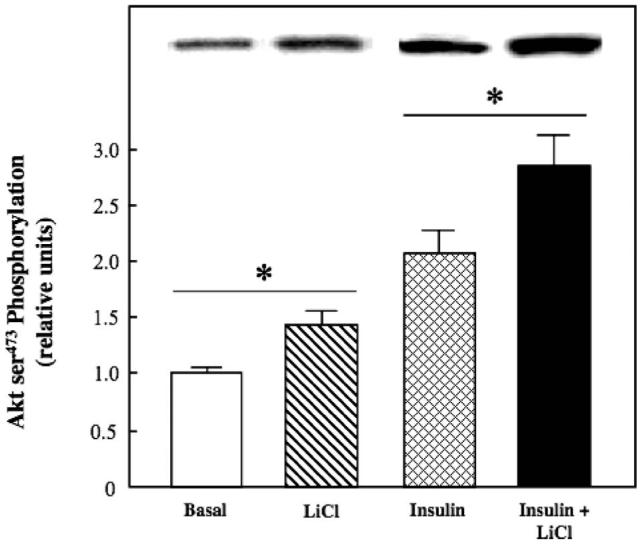
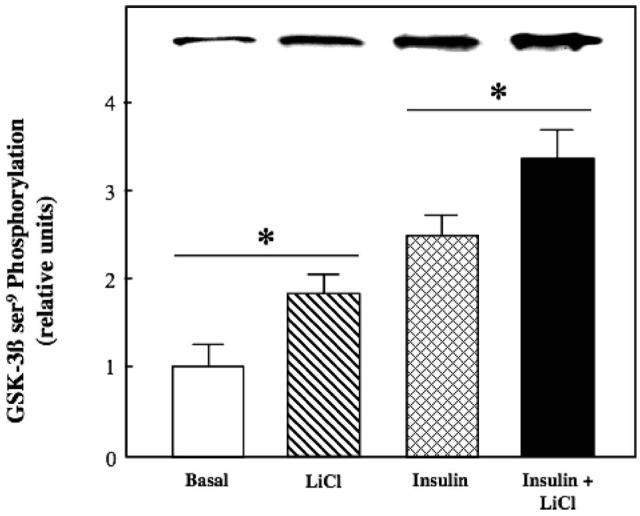
We next assessed the action of lithium to modulate the functional status of several critical distal and proximal elements of the insulin signalling pathway. Lithium, alone or in combination with insulin, did not affect tyrosine phosphorylation of IR-β (Figure 3). In contrast, lithium by itself enhanced Akt ser473 phosphorylation by 40% (Figure 4) and GSK-3β ser9 phosphorylation by 88% (the latter effect reflecting a decrease in GSK-3 activity) (Figure 5) (both p < 0.05). Moreover, lithium significantly potentiated (p < 0.05) the stimulatory effects of insulin on Akt ser473 phosphorylation by 39% and on GSK-3β ser9 phosphorylation by 36%.
Figure 3.
Effect of lithium on basal and insulin-stimulated tyrosine phosphorylation of insulin receptor in insulin-resistant skeletal muscle. Soleus muscle from obese Zucker rats was incubated in the absence or presence of 10 mM LiCl without or with 5 mU/ml insulin, and overall tyrosine phosphorylation of the β-subunit of the insulin receptor was assessed as described in Materials and methods. Representative bands from the autoradiograph are displayed at the top of the figure. Values are means±SE for 4-5 muscles per group.
Figure 4.
Effect of lithium on basal and insulin-stimulated Akt phosphorylation in insulin-resistant skeletal muscle. Soleus muscle from obese Zucker rats was incubated in the absence or presence of 10 mM LiCl without or with 5 mU/ml insulin, and ser473 phosphorylation was assessed as described in Materials and methods. Representative bands from the autoradiograph are displayed at the top of the figure. Values are means±SE for 4-5 muscles per group. *p < 0.05, LiCl vs. basal or insulin + LiCl vs. insulin, by paired Student's t-test.
Figure 5.
Effect of lithium on basal and insulin-stimulated glycogen synthase kinase-3 phosphorylation in insulin-resistant skeletal muscle. Soleus muscles from obese Zucker rats were incubated in the absence or presence of 10 mM LiCl without or with 5 mU/ml insulin, and ser9 phosphorylation on GSK-3β was assessed as described in Materials and methods. Representative bands from the autoradiograph are displayed at the top of the figure. Values are means±SE for 4-5 muscles per group. *p < 0.05, LiCl vs. basal or insulin + LiCl vs. insulin, by paired Student's t-test.
Lithium action on p38 MAPK phosphorylation in insulin-resistant muscle
Lithium treatment induced a robust increase in thr180/tyr182 phosphorylation of p38 MAPK both in the absence (100%) and presence (88%) (both p < 0.05) of insulin (Figure 6). Insulin alone did not enhance p38 MAPK phosphorylation in the insulin-resistant skeletal muscle, in agreement with our previous results from insulin-sensitive muscle (O'Keefe et al., 2004; Harrell et al., 2007). The lithium treatment did not significantly alter the phosphorylation state of other stress-activated kinases, including AMPKα and JNK1/2 (data not shown).
The specific role of the p38 MAPK activation in the enhancement of glucose transport activity by lithium in insulin-resistant skeletal muscle was further investigated using A304000, a selective inhibitor of p38 MAPK (Somwar et al., 2002; Kim et al., 2006). A304000 (10 μM) itself had no significant effect on basal glucose transport (Figure 7, left panel). In contrast, the inhibition of p38 MAPK with A304000 completely prevented the lithium-induced increase in basal glucose transport activity (Figure 7, left panel). This concentration of A304000 prevented 65% (p < 0.05) of the lithium-induced enhancement of p38 MAPK phosphorylation (Figure 8, upper left panel) without altering Akt ser473 phosphorylation (Figure 8, lower left panel). The p38 MAPK inhibition significantly reduced (37%, p < 0.05) insulin-stimulated glucose transport activity in the soleus of the obese Zucker rat (Figure 7, right panel), supporting a role of p38 MAPK activation in the insulin-dependent regulation of glucose transport in insulin-resistant skeletal muscle. Moreover, the p38 MAPK inhibitor reduced by 42% (p < 0.05) the markedly enhanced glucose transport activity in the presence of insulin and lithium in combination (Figure 7, right panel). Interestingly, there was only a trend (p < 0.20) for a 24% reduction due to A304000 in the enhanced p38 MAPK phosphorylation caused by lithium and insulin in combination (Figure 8, upper right panel). Stimulation of Akt ser473 phosphoryaltion by insulin alone or by insulin in combination with lithium was not affected by the p38 MAPK inhibitor (Figure 8, lower right panel). Taken together, these data support the concept that the engagement of p38 MAPK plays a critical role in the action of lithium on the glucose transport system in insulin-resistant skeletal muscle. However, factors other than p38 MAPK alone must underlie the interaction of lithium and insulin in the stimulation of glucose transport in this insulin-resistant skeletal muscle.
Discussion
In the present investigation, we have demonstrated that the alkali metal lithium increases basal glucose transport (Figure 1) and glycogen synthesis (Figure 2) in isolated soleus muscle of the female obese Zucker rat, a rodent model of pre-diabetes and skeletal muscle insulin resistance. Importantly, we have documented for the first time a lithium-induced potentiation of insulin action on these parameters in insulin-resistant skeletal muscle, with the increases in glucose transport activity and glycogen synthesis elicited by lithium and insulin far exceeding the theoretical additive effects of the individual compounds. A further novel finding of our study was that these actions of lithium on the glucose transport system in insulin-resistant skeletal muscle are associated with increased phosphorylation of Akt (ser473) (Figure 3) and GSK-3β (ser9) (Figure 4), both in the absence and presence of insulin. The increase in Akt ser473 phosphorylation could directly mediate the enhancement of glucose transport activity elicited by lithium, as this step in the insulin signalling pathway has been show to be critical for the normal action of insulin to stimulate glucose transport activity (Kohn et al., 1996; Hadjuch et al., 1998; Ueki et al., 1998). On the other hand, the increased serine phosphorylation of GSK-3β is likely directly related to the lithium-induced enhancement of glycogenesis, as the more phosphorylated form of GSK-3β is less active, thereby allowing glycogen synthase to be activated (Henriksen et al., 2003) and facilitate glucose incorporation into glycogen (Roach, 1990; Zhang et al., 1993). Interestingly, lithium had no effect on tyrosine phosphorylation of IR, in agreement with our previous observations in insulin-sensitive skeletal muscle of the female lean Zucker rat (Harrell et al., 2007).
An additional major finding of the present study is that lithium induced a robust enhancement of p38 MAPK thr180/tyr182 phosphorylation (Figure 5) in the insulin-resistant skeletal muscle. Moreover, the selective inhibition of p38 MAPK by A304000 completely blocked the lithium-induced enhancement of glucose transport activity (Figure 6) and significantly reduced insulin-stimulated glucose transport activity (Figure 6) in this tissue. Much controversy surrounds the involvement of p38 MAPK in the action of insulin on the glucose transport process (Furtado et al., 2003; Antonescu et al., 2005). Nevertheless, our findings in the present study confirm results from previous studies in cell lines (MacAuley et al., 2003) and insulin-sensitive mammalian skeletal muscle (Harrell et al., 2007) that p38 MAPK activity is essential in the mediation of lithium action on glucose transport, and underscores the fact that this p38 MAPK-dependent mechanism is intact in insulin-resistant skeletal muscle. However, the exact molecular mechanisms linking p38 MAPK activation and modulation of the glucose transport system remain unclear.
A single previous investigation has assessed the impact of lithium on glucose metabolism in skeletal muscle of the obese Zucker rat (Fürnsinn et al., 1997). In that study, the maximal in vitro effects of lithium chloride on glucose transport and glycogen synthesis in soleus muscle of male obese Zucker rats were directly compared with those of insulin. The maximal effect of insulin on glucose transport was substantially greater than the effect of lithium (Fürnsinn et al., 1997), in contrast with the present results in soleus muscle from female obese Zucker rats showing essentially equal effects of insulin and lithium on this variable (Figure 1). Moreover, Fürnsinn et al. (1997) showed that insulin and lithium elicited equal maximal stimulation of glycogen synthesis, whereas in the present investigation the maximal effect of insulin on glycogenesis was markedly greater than that of lithium (Figure 2). At present, aside from the obvious gender difference between the studies, the underlying cause for these variant results is not clear. It should be noted that the study of Fürnsinn et al. (1997) did not investigate the potential interactions between insulin and lithium in the insulin-resistant skeletal muscle, further underscoring the novel results of the present study.
A comparison of lithium action on insulin-sensitive skeletal muscle from lean Zucker rats (Harrell et al., 2007) and insulin-resistant skeletal muscle from obese Zucker rats (present study) reveals many similarities and one striking difference. Lithium activates glucose transport, glycogen synthesis, GSK-3β phosphorylation, and p38 MAPK phosphorylation in both insulin-sensitive and insulin-resistant skeletal muscle. However, in insulin-resistant skeletal muscle, lithium increases Akt phosphorylation (Figure 4), whereas lithium has no effect on this variable in insulin-sensitive skeletal muscle. One possible explanation for this difference is that insulin action on Akt phosphorylation is impaired in muscle of the obese Zucker rat due to upstream signalling defects (Anai et al., 1998; Saengsirsuwan et al., 2004), and lithium action on upstream signalling factors could ameliorate this defect at the level of Akt. Indeed, GSK-3 is overactive in skeletal muscle of the obese Zucker rat, leading to impaired IRS-1 activity and downstream activation of Akt, defects that can be modulated with selective GSK-3 inhibition (Dokken et al., 2005). As one effect of lithium is to act as a GSK-3 inhibitor (though non-selectively), this would allow lithium-mediated disinhibition of both IRS-1 and Akt. This potential action of lithium on insulin signalling clearly warrants further investigation in insulin-resistant skeletal muscle.
Despite the well-documented stimulatory effects of lithium on glucose transport and glycogen synthesis and the potential of lithium as a GSK-3 inhibitor, several confounding issues would limit the use of the lithium as a viable intervention in the treatment of human insulin resistance. For example, oral administration of lithium would make this alkali metal available to all of the tissues of the body, and the actions of lithium as a GSK-3 inhibitor and via GSK-3-independent mechanisms could have undesirable negative side effects on cell differentiation and proliferation (Henriksen & Dokken, 2006). In addition, the psychoactive impact of lithium (Van de Velde & Gordon, 1969; Vornik & Brown, 2006) would also limit its safety in insulin-resistant subjects with normal mental health status. Nonetheless, the experimental application of lithium continues to be highly valuable in the elucidation of molecular mechanisms underlying insulin resistance and for the identification of potential targets for pharmaco-therapeutic intervention and clinical applications.
In summary, in the present investigation we have demonstrated in isolated, insulin-resistant skeletal muscle of the obese Zucker rat that the alkali metal lithium increases basal glucose transport activity and glycogen synthesis, and significantly potentiates the action of insulin on these processes. Moreover, these effects of lithium are associated with enhanced serine phosphorylation of Akt and GSK-3ß, but not tyrosine phosphorylation of IR-β. Importantly, we have shown in the insulin-resistant skeletal muscle of the obese Zucker rat that the lithium-induced activation of glucose transport is dependent on the engagement of the stress-activated kinase p38 MAPK. The present results support the continued use of lithium to target pharmacologically specific defects in insulin signalling for the treatment of insulin resistance in mammalian skeletal muscle.
Acknowledgements
The study was supported by NIH grant DK063967 (to E.J.H.).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Anai M, Funaki M, Ogihara T, Terasaki J, Inukai K, Katagiri H, Fukushima Y, Yazaki Y, Kikuchi M, Oka Y, et al. Altered expression levels and impaired steps in pathways to phosphatidylinositol-3-kinase activation via insulin receptor substrates 1 and 2 in Zucker fatty rats. Diabetes. 1998;47:13–23. doi: 10.2337/diab.47.1.13. [DOI] [PubMed] [Google Scholar]
- Antonescu CN, Huang C, Niu W, Liu Z, Eyers PA, Heidenreich KA, Bilan PJ, Klip A. Reduction of insulin-stimulated glucose uptake in L6 myotubes by the protein kinase inhibitor SB203580 in independent of p38MAPK activity. Endocrinology. 2005;146:3773–81. doi: 10.1210/en.2005-0404. [DOI] [PubMed] [Google Scholar]
- Crettaz M, Prentki M, Zaninetti D, Jeanrenaud B. Insulin resistance in soleus muscle from obese Zucker rats:involvement of several defective sites. Biochem J. 1980;186:525–34. doi: 10.1042/bj1860525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Ilouz R. Challenges and opportunities with glycogen synthase kinase-3 inhibitors for insulin resistance and type 2 diabetes treatment. Expert Opin Invest Drugs. 2003;12:1511–19. doi: 10.1517/13543784.12.9.1511. [DOI] [PubMed] [Google Scholar]
- Etgen GJ, Wilson CM, Jensen J, Cushman SW, Ivy JL. Glucose transport and cell surface GLUT-4 protein in skeletal muscle of the obese Zucker rat. Am J Physiol Endocrinol Metab. 1996;271:E294–E301. doi: 10.1152/ajpendo.1996.271.2.E294. [DOI] [PubMed] [Google Scholar]
- Fürnsinn C, Noe C, Herdlicka R, Roden M, Nowotny P, Leighton B, Waldhäsl More marked stimulation by lithium than insulin of the glycogenic pathway in rat skeletal muscle. Am J Physiol Endocrinol Metab. 1997;273:E514–E520. doi: 10.1152/ajpendo.1997.273.3.E514. [DOI] [PubMed] [Google Scholar]
- Furtado LM, Poon V, Klip A. GLUT4 activation: some thoughts on possible mechanisms. Acta Physiol Scand. 2003;178:287–96. doi: 10.1046/j.1365-201X.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- Hajduch E, Alessi DR, Hemmings BA, Hundal HS. Constitutive activation of protein kinase Bα by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes. 1998;47:1006–13. doi: 10.2337/diabetes.47.7.1006. [DOI] [PubMed] [Google Scholar]
- Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol. 1994;76:1862–7. doi: 10.1152/jappl.1994.76.2.979. [DOI] [PubMed] [Google Scholar]
- Harrell NB, Teachey MK, Gifford NJ, Henriksen EJ. Essential role of p38 MAPK for activation of skeletal muscle glucose transport by lithium. Arch Physiol Biochem. 2007;113:221–7. doi: 10.1080/13813450701783158. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol Endocrinol Metab. 1990;259:E593–E598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Dokken BB. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Current Drug Targets. 2006;7:1435–42. doi: 10.2174/1389450110607011435. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Jacob S. Effects of captopril on glucose transport activity in skeletal muscle of obese Zucker rats. Metabolism. 1995;44:267–72. doi: 10.1016/0026-0495(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Kinnick TR, Teachey MK, O'Keefe MP, Ring D, Johnson KW, Harrison SD. Modulation of muscle insulin resistance by selective glycogen synthase kinase-3 inhibition in Zucker Diabetic Fatty rats. Am J Physiol Endocrinol Metab. 2003;284:E892–E900. doi: 10.1152/ajpendo.00346.2002. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Teachey MK. Short-term in vitro inhibition of glycogen synthase kinase-3 potentiates insulin signalling in skeletal muscle of Zucker Diabetic Fatty rats. Metabolism. 2007;56:931–938. doi: 10.1016/j.metabol.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ, Tischler ME, Johnson DG. Increased response to insulin of glucose metabolism in the 6-day unloaded rat soleus muscle. J Biol Chem. 1986;261:10707–12. [PubMed] [Google Scholar]
- Kim JS, Saengsirisuwan V, Sloniger JA, Teachey MK, Henriksen EJ. Stimulation of muscle glucose transport by an oxidant stress: roles of insulin signalling and p38 MAP kinase. Free Rad Biol Med. 2006;41:818–24. doi: 10.1016/j.freeradbiomed.2006.05.031. [DOI] [PubMed] [Google Scholar]
- King PA, Horton ED, Hirshman MF, Horton ES. Insulin resistance in obese Zucker rat (fa/fa) is associated with a failure of glucose transporter translocation. J Clin Invest. 1993;90:1568–75. doi: 10.1172/JCI116025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulated glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–8. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- Konrad D, Somwar R, Sweeney G, Yaworsky K, Hayashi M, Ramlal T, Klip A. The anti-hyperglycemic drug α-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation. Diabetes. 2001;50:1464–71. doi: 10.2337/diabetes.50.6.1464. [DOI] [PubMed] [Google Scholar]
- MacAulay K, Hajduch E, Blair A, Coghlan MP, Smith SA, Hundal HS. Use of lithium and SB-415286 to explore the role of glycogen synthase kinase-3 in the regulation of glucose transport and glycogen synthase. Eur J Biochem. 2003;270:3829–38. doi: 10.1046/j.1432-1033.2003.03777.x. [DOI] [PubMed] [Google Scholar]
- Mathe D. Dyslipidemia and diabetes: animal models. Diab Metabol. 1995;21:106–11. [PubMed] [Google Scholar]
- O'Keefe MP, Perez FR, Kinnick TR, Tischler ME, Henriksen EJ. Development of whole-body and skeletal muscle insulin resistance after one day of hind limb suspension. Metabolism. 2004;53:1215–22. doi: 10.1016/j.metabol.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Parker PJ, Caudwell FB, Cohen P. Glycogen synthase from rabbit skeletal muscle: effect of insulin on the state of phosphorylation of the seven phosphoserine residues in vivo. Eur J Biochem. 1983;130:227–34. doi: 10.1111/j.1432-1033.1983.tb07140.x. [DOI] [PubMed] [Google Scholar]
- Roach PJ. Control of glycogen synthase by hierarchal protein phosphorylation. FASEB J. 1990;4:2961–8. [PubMed] [Google Scholar]
- Saengsirisuwan V, Perez FR, Sloniger JA, Maier T, Henriksen EJ. Interactions of exercise training and R-(+)-alpha-lipoic acid on insulin signalling in skeletal muscle of obese Zucker rats. Am J Physiol Endocrinol Metab. 2004;287:E529–E536. doi: 10.1152/ajpendo.00013.2004. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Kahn BB. Glucose transporters and insulin action. N Eng J Med. 1999;341:248–57. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- Somwar R, Koterski S, Sweeney G, Sciotti R, Djuric S, Berg C, Trevillyan J, Scherer PE, Rondinone CM, Klip A. A dominant-negative p38 MAPK mutant and novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3-L1 adipocytes without affecting GLUT4 translocation. J Biol Chem. 2002;277:50386–95. doi: 10.1074/jbc.M205277200. [DOI] [PubMed] [Google Scholar]
- Somwar R, Perreault M, Kapur S, Tahu C, Sweeney G, Ramlal T, Kim DY, Keen J, Cote CH, Klip A, et al. Activation of p38 mitogen-activated protein kinase α and ß by insulin and contraction in rat skeletal muscle. Potential role in the stimulation of glucose transport. Diabetes. 2000;49:1794–800. doi: 10.2337/diabetes.49.11.1794. [DOI] [PubMed] [Google Scholar]
- Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3-L1 adipocytes and L6 myotubes. J Biol Chem. 1999;274:10071–8. doi: 10.1074/jbc.274.15.10071. [DOI] [PubMed] [Google Scholar]
- Tabata I, Schluter J, Gulve EA, Holloszy JO. Lithium increases susceptibility of muscle glucose transport to stimulation by various agents. Diabetes. 1994;43:903–7. doi: 10.2337/diab.43.7.903. [DOI] [PubMed] [Google Scholar]
- Ueki K, Yamamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering BMT, Coffer PJ, Komuro I, Akanuma Y, Yazaki Y, et al. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–22. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- Van de Velde C, Gordon M. Manic-depressive illness, diabetes mellitus, and lithium carbonate. Arch Gen Psych. 1969;21:478–85. doi: 10.1001/archpsyc.1969.01740220094011. [DOI] [PubMed] [Google Scholar]
- Vornik LA, Brown ES. Management of comorbid bipolar disorder and substance abuse. J Clin Psych. 2006;67(Suppl 7):24–30. [PubMed] [Google Scholar]
- Wagman AS, Johnson KW, Bussiere DE. Discovery and development of GSK3 inhibitors for the treatment of type 2 diabetes. Curr Pharmaceut Design. 2004;10:1105–37. doi: 10.2174/1381612043452668. [DOI] [PubMed] [Google Scholar]
- Zhang W, DePaoli-Roach AA, Roach PJ. Mechanisms of multisite phosphorylation and inactivation of rabbit muscle glycogen synthase. Arch Biochem Biophys. 1993;304:219–25. doi: 10.1006/abbi.1993.1342. [DOI] [PubMed] [Google Scholar]
- Zierath JR, Krook A, Wallberg-Henriksson H. Insulin action and insulin resistance in human skeletal muscle. Diabetologia. 2000;43:821–35. doi: 10.1007/s001250051457. [DOI] [PubMed] [Google Scholar]