Abstract
The demands of sustaining high levels of neurobehavioral performance during space operations necessitate precise scheduling of sleep opportunities in order to best preserve optimal performance. We report here the results of the first split-sleep, dose-response experiment involving a range of sleep/wake scenarios with chronically reduced nocturnal sleep, augmented with a diurnal nap. To characterize performance over all combinations of split sleep in the range studied, we used response surface mapping methodology. Waking neurobehavioral performance was studied in N=90 subjects each assigned to one of 18 sleep regimens consisting of a restricted nocturnal anchor sleep period and a diurnal nap. Psychomotor vigilance task performance and subjective assessments of sleepiness were found to be primarily a function of total time in bed per 24 h regardless of how sleep was divided among nocturnal anchor sleep and diurnal nap periods. Digit symbol substitution task performance was also found to be primarily a function of total time in bed per 24 h; however, accounting for nocturnal sleep duration and nap duration separately provided a small but significant enhancement in the variance explained. The results suggest that reductions in total daily sleep result in a near-linear accumulation of impairment regardless of whether sleep is scheduled as a consolidated nocturnal sleep period or split into a nocturnal anchor sleep period and a diurnal nap. Thus, split sleep schedules are feasible and can be used to enhance the flexibility of sleep/work schedules for space operations involving restricted nocturnal sleep due to mission-critical task scheduling. These results are generally applicable to any continuous industrial operation that involves sleep restriction, night operations, and shift work.
Keywords: chronic sleep restriction, split sleep schedules, space flight, anchor sleep, napping, sleep physiology, neurobehavioral performance, subjective sleepiness, psychomotor vigilance task, response surface mapping, mixed-effects regression
1. INTRODUCTION
Sustaining high levels of neurobehavioral performance during extended space operations is of paramount importance to astronaut safety and the achievement of mission goals. Future space missions are expected to be longer in duration with continuous operations on the International Space Station, extended lunar missions, and a multi-year mission to Mars. The demands of sustaining high levels of neurobehavioral performance coupled with mission-critical time constraints necessitate precise scheduling of sleep opportunities in order to best preserve optimal performance. Objective evidence from virtually all NASA space missions indicates that astronaut sleep is restricted in space flight, averaging between 4h and 6.5h per day, as a result of endogenous disturbances of sleep (e.g., motion sickness, circadian desynchrony), environmental disruptions, and frequently, curtailment of sleep due to the work demands [1]. Ground based experiments indicate that cognitive performance deficits progressively worsen (i.e., accumulate) over consecutive days when sleep is restricted to levels experienced by astronauts [2, 3]. The detrimental consequences of sleep restriction manifest in a range of critical functions including increased lapses of attention, degradation of response times, deficits in complex problem solving, reduced learning, increased negative mood, and disruption of essential endocrine, metabolic, and immune responses [4, 5].
Scheduling time for sleep during space flight is largely determined by the operational needs of the mission. However, sleep and wakefulness are regulated by a complex neurobiology involving interacting homeostatic and circadian mechanisms [6–8]. Effective sleep/wake schedules for space operations must account for this fundamental neurobiology. This requires precise knowledge of the relationship between the timing and duration of sleep and the level of waking neurobehavioral performance capability. Such knowledge can only be obtained by performing experiments that examine waking neurobehavioral performance while systematically varying sleep duration and sleep timing.
We investigated a range of sleep/wake scenarios involving chronically reduced nocturnal sleep, augmented with a daily diurnal nap, and studied the effects on waking neurobehavioral functions. The experiment had two goals: (1) Determine whether split sleep schedules with reduced total daily sleep time can result in increased total wake time while preventing cumulative reductions in waking cognitive function; (2) Determine whether split sleep schedules are at least equally effective in preventing cumulative reductions in waking cognitive function as monophasic sleep schedules of the same daily total sleep time. Although the selection of sleep schedules examined was based on observed sleep durations during NASA space missions the results generally apply to any continuous industrial operation that involves sleep restriction, night operations, and shift work such as maritime operations, military operations, power generation, manufacturing, etc. In order to characterize performance over all combinations of split sleep in the range studied, we used response surface mapping (RSM)—a technique commonly used in chemical engineering to optimize industrial processes. RSM provides a means to assess relationships over a continuum by conducting a discrete set of dose-response experiments.
We applied RSM previously to investigate the efficiency of physiological sleep obtained across the 18 split sleep schedules [9]. It was found that in terms of total amount of physiologic sleep time, it did not substantively matter whether the sleep was chronically placed nocturnally or split between nocturnal anchor sleep periods and daytime naps. Demonstrating that split sleep schedules are feasible in terms of obtaining physiological sleep provided a foundation for the current analysis, which examines whether some of these schedules may be advantageous to mitigate neurobehavioral performance impairment in the face of limited time for sleep. We report here the results of the first split sleep dose-response experiment involving 18 sleep regimens consisting of a restricted nocturnal anchor sleep period and a diurnal nap.
2. METHODS
2.1. Study Design and Participants
A total of 90 healthy adult subjects (52 males, 38 females; mean age 29.5 y, age range 21–49 y) participated in the experiment. Subjects were screened to ensure they had no medical, psychiatric, or sleep-related disorders and were drug-free. This was determined by history, physical examination and psychological questionnaires, and by blood and urine laboratory tests and toxicological screening. Subjects could not have worked regular or rotating shift work within the past 2 years, and they could not have traveled across time zones in the 3 months prior to the experiment. They were required to be in bed for approximately 8 h daily during the week preceding the study, as verified by wrist actigraphy [10] combined with daily diary reports and time-stamped phone records for time to bed and time awake. The Institutional Review Board of the University of Pennsylvania reviewed and approved the study, and each subject gave written informed consent. Subjects were compensated for their participation in the study.
Subjects participated in a 14-day laboratory study involving strict schedules for time in bed (TIB). The laboratory experiment began with two baseline nights, each involving 8.2 h TIB (21:54 to 6:06). Subjects were then randomly assigned to one of 18 sleep-restriction conditions, each involving a specific sleep regimen that was maintained for 10 consecutive days—see Table 1. The final day of the study consisted of a recovery sleep period of 14 h TIB (21:54 to 11:54).
Table 1.
Conditions studied in the split-sleep, dose-response experiment. The table shows the total time in bed (TIB) per 24 h across the 10 sleep restriction days, as a function of nocturnal anchor sleep TIB and diurnal nap sleep TIB, for each of the 18 conditions.
| Diurnal nap sleep TIB (h) |
Nocturnal anchor sleep TIB (h) | |||
|---|---|---|---|---|
| 4.2 | 5.2 | 6.2 | 8.2 | |
| 0.0 | 4.2 | 5.2 | 6.2 | 8.2 |
| 0.4 | 4.6 | 5.2 | 6.6 | * |
| 0.8 | 5.0 | 6.0 | 7.0 | * |
| 1.2 | 5.4 | 6.4 | 7.4 | * |
| 1.6 | 5.8 | 6.8 | * | * |
| 2.0 | 6.2 | 7.2 | * | * |
| 2.4 | 6.6 | * | * | * |
Condition not studied.
The 10-day sleep restriction assignments involved one of four nocturnal anchor sleep conditions: 4.2, 5.2, 6.2, or 8.2 h TIB centered around 02:00—and one of seven diurnal nap sleep conditions: 0.0, 0.4, 0.8, 1.2, 1.6, 2.0, 2.4 h TIB centered around 14:00. The term anchor-sleep is taken from the work of Minor and Waterhouse to mean the habitual sleep period that is the intermediary by which many zeitgebers (light-dark; eating-fasting; social influences) exert their effects upon the internal pacemaker [11, 12].These different anchor and nap sleep durations were crossed to yield a total of four anchor-sleep-only conditions and fourteen anchor-plus-nap-sleep or split sleep conditions—see Table 1. Five subjects were assigned to each of the experimental conditions. Since our study was not focused on the effects of any specific sleep regimen but rather on the response surface map formed by the different experimental conditions, our analyses had an effective sample size of N = 90.
Throughout the entire 14-day experimental period, subjects remained in an environmentally controlled laboratory, free of external time cues, and were under continuous physiological and behavioral monitoring. During all scheduled waking periods they were kept awake by performance requirements and by behavioral/social interaction. They underwent neurobehavioral assessments of cognitive performance, mood and symptom complaints every 2 h throughout all waking periods every day. Between test bouts they were allowed to read, watch movies, and interact with laboratory staff to help them stay awake, but no vigorous activities were permitted. They were in less than 50 lux of light during all scheduled wake times. During scheduled sleep times, all lights were turned off.
2.2. Measurements
Compliance with prescribed sleep schedules was verified by polysomnography for both the nocturnal sleep periods and diurnal nap sleep periods during the baseline days and the 10 sleep-restriction days, except for the diurnal nap sleep periods on days 2 and 7 and the subsequent nocturnal sleep periods on days 3 and 8 (these two 24 h periods were without electrodes to reduce any skin irritation and to permit subjects to shower). The polysomnographic data of this study have been reported elsewhere [9].
Subjects underwent neurobehavioral assessments every 2 h during scheduled wakefulness. For all conditions except the control condition (8.2 h anchor sleep per day with no daytime nap) this amounted to 10 test bouts per day. These test bouts were scheduled at the following times of day: 4:10, 6:10, 8:10, 10:10, 12:10, 14:10, 16:10, 18:10, 20:10, and 22:10. Subjects in the control condition were not tested at 4:10 and 22:20 and had their 6:10 test bout immediately following their nocturnal sleep period. Thus, subjects in the control condition had two fewer neurobehavioral test bouts per day. Subjects in conditions with 5.2 h and 6.2 h anchor sleep periods had their 4:10 test bout immediately following their nocturnal sleep period and their 6:10 test bout within 90 minutes of awakening. Subjects in conditions with diurnal naps had their 14:10 test bout immediately following their scheduled nap sleep period.
The psychomotor vigilance task was included in the neurobehavioral assessment battery as a measure of behavioral alertness. The psychomotor vigilance task is a sustained-attention reaction-time task with a random inter-stimulus interval of 2–10 s. Lapses (reaction times greater than 500 ms) were counted per 10 minute test to measure impairments in behavioral alertness. The digit symbol substitution task was included among the neurobehavioral tasks as a measure speed and accuracy of cognitive throughput. This subject-paced task involves the matching of digits (0–9) to symbols (circle, triangle, etc.). The number of correct matches during a 1.5 minute interval was counted to measure cognitive throughput. The Stanford sleepiness scale was also included among the neurobehavioral tasks. Subjects provided a subjective assessment of their sleepiness on a 7-point scale at the beginning of each neurobehavioral assessment battery.
Daily values for each performance task were calculated by averaging scores from six performance test bouts (8:10, 10:10, 12:10, 16:10, 18:10, and 20:10) to assess the profiles of sleepiness and performance across days of sleep restriction. Other test bouts were excluded in the primary analysis to avoid inclusion of sleep inertia effects or because the test bout was not administered in all conditions. To test the consistency of the results when test bouts that were administered shortly after waking were included, the analysis was repeated using daily values calculated by averaging scores from all performance test bouts (6:10, 8:10, 10:10, 12:10, 14:10, 16:10, 18:10 and 20:10) that were administered in all conditions. At baseline, there were no significant differences among the experimental conditions for psychomotor vigilance task performance (one-way ANOVA, F17,72 =0.85, p=0.64), digit symbol substitution task performance, (one-way ANOVA, F17,72 =1.43, p=0.15), or Stanford sleepiness scale rating (one-way ANOVA, F17,72 =0.82, P=0.66).
2.3. Response surface mapping
Performance scores were averaged over each sleep restriction day and normalized for each subject by subtracting their own baseline scores (day 2) to measure performance change over days. Non-linear mixed-effects regression models were applied to the performance scores across the ten sleep restriction days. This analysis simultaneously accounted for changes over days and for systematic differences among subjects. Covariates for age (linear and quadratic) and gender were included, as well as a normally distributed random effect over subjects. Numerical implementation was done in SAS (Version 9.1, SAS Institute Inc., Cary, NC) using the procedure NLMIXED. For a more complete treatment of Non-linear mixed-effects regression models the reader is referred to the literature [13–15].
Response surface mapping typically deals with the optimization of reactions and mixtures where the variable of interest is a function of several predictor variables. In the case where the relationship between the variable of interest and the predictor variables is not fully understood empirical response surface models are developed and tested against experimental data [16]. In the current context response surface maps are developed where the variables of interest include measures of neurobehavioral performance and the predictor variables are nocturnal sleep duration (TIB ) and diurnal nap sleep duration (TIB). As it is a mixture-type response surface mapping problem with two components it therefore lends itself to graphical representation on a 3 dimension Cartesian grid. For each variable of interest a series of nested models are developed that test a specific hypothesis about the relationship between the variable of interest and nocturnal sleep and diurnal nap sleep duration. Hypothesis testing is accomplished using the test for nested hypotheses using the likelihood ratio criterion. The likelihood ratio test involves taking the ratio of the negative 2 log likelihood of the restricted model given the observed data (maximum likelihood estimate generated in SAS using PROC NLMIXED) over the negative 2 log likelihood of the full model. This ratio is asymptotically Chi squared with degrees of freedom equal to the difference in the number of parameters between the full model and the restricted model [16].
The analyses began with a model that accounts for condition-specific slopes, which in essence constituted a two-way analysis of variance (ANOVA) over the different nocturnal anchor sleep and nap sleep conditions:
| (1) |
where t denotes days of sleep restriction. The parameter β is a normally distributed random variable with a condition-specific mean representing the rate of change of neurobehavioral performance across sleep restriction days. The parameter θ represents the degree of curvature and quantifies the extent to which the effect of days is non-linear [2]. This approach allowed for response surface mapping (RSM) of results—that is, the construction of three-dimensional graphs of the slopes of the condition-specific nonlinear changes over days (on the “z-axis”) versus the different anchor sleep TIB ( “x-axis”) and nap sleep TIB (“y-axis”) assignments.
Following estimation of the full model with condition-specific non-linear changes over days, various reduced versions of the model were fitted in order to establish systematic patterns among the different conditions. One such reduced model assumed that there was no interaction between anchor and nap sleep:
| (2) |
Here, the rate of change of neurobehavioral performance across sleep restriction days is no longer idiosyncratic for each condition, but depends on the TIB for anchor sleep (through β) independent of nap sleep duration and vice versa on the TIB for nap sleep (through γ) independent of anchor sleep duration.
A further reduced model assumed linearity over conditions:
| (3) |
where β and γ are now rate constants (e.g., for every hour of additional anchor sleep, the rate of change of neurobehavioral performance across sleep restriction days is altered by β); and α is an overall intercept.
The most reduced model we considered substituted anchor TIB and nap TIB by the combined TIB during the 24 h of the day:
| (4) |
where the parameter δ represents the rate of change of neurobehavioral performance across days as a function of total daily TIB. Involving not two independent variables (anchor and nap sleep) but only one, this model no longer required response surface mapping over conditions, but reduced to a univariate regression which can be plotted in conventional two-dimensional graphs (with the combined TIB as the only independent variable).
Confidence intervals were estimated by means of bootstrapping [17]. This was implemented numerically in SAS by re-sampling 1,000 times with replacement. The bootstrap 95% confidence intervals were assessed by taking the 2.5th and 97.5th percentiles of the resulting distributions.
Non-linear regression has the benefit of accounting for non-linearities in the effect of sleep restriction over experiment days. The value of the curvature parameter θ estimated from the data was found to be 0.81 ± 0.05 for psychomotor vigilance performance, 0.50 ± 0.03 for digit symbol substitution task performance, and 0.44 ± 0.04 for Stanford sleepiness scale ratings of subjective sleepiness (estimate ± s.e.). These values indicate a near-linear relationship of performance changes over days for psychomotor vigilance performance and a more non-linear relationship over days for digit symbol substitution task performance and Stanford sleepiness scale subjective sleepiness ratings. This is similar to what was observed in earlier research [2]. The value of θ is not of particular interest in this paper, and will therefore not be further discussed.
3. RESULTS
3.1. Psychomotor vigilance performance
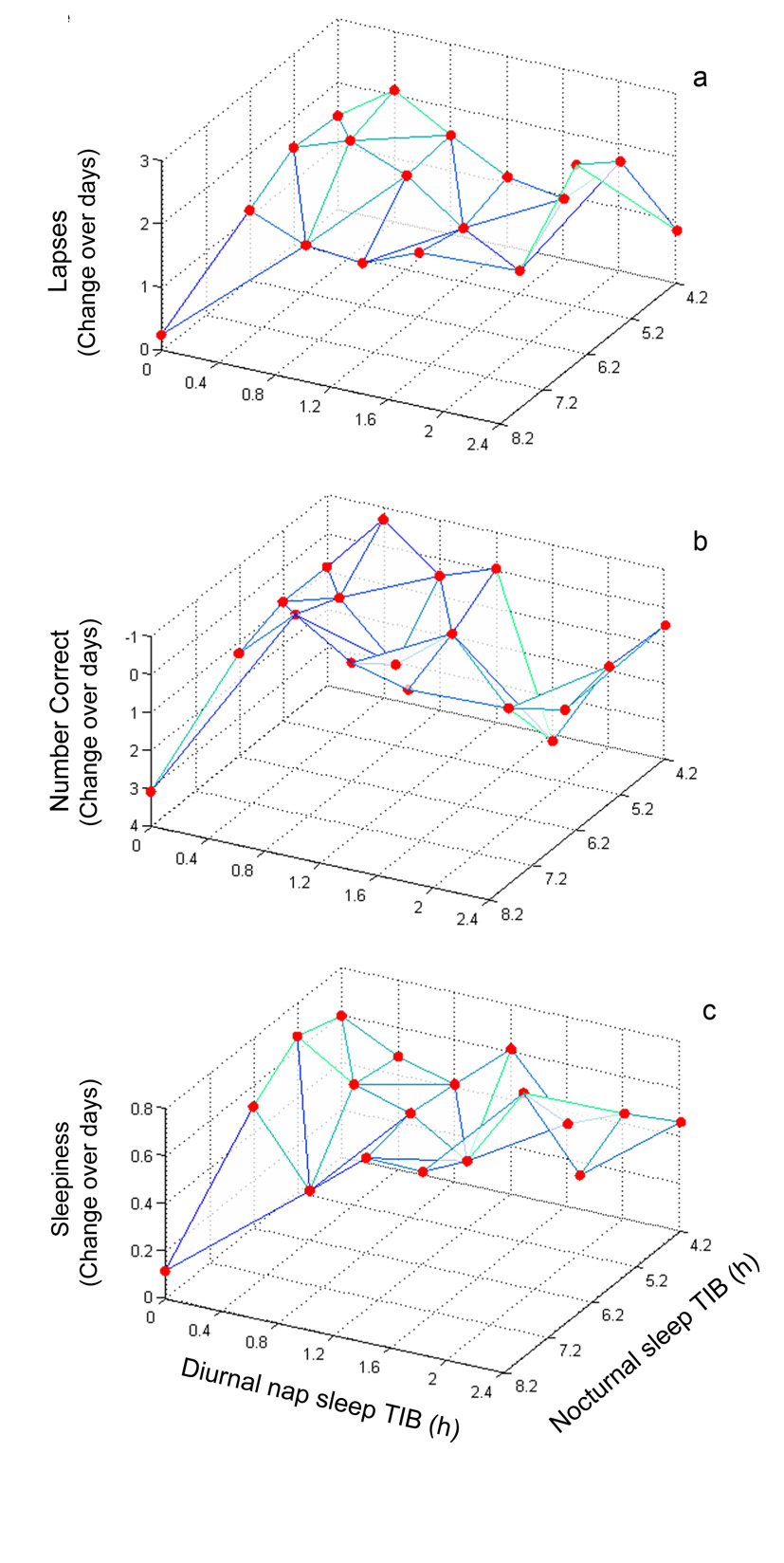
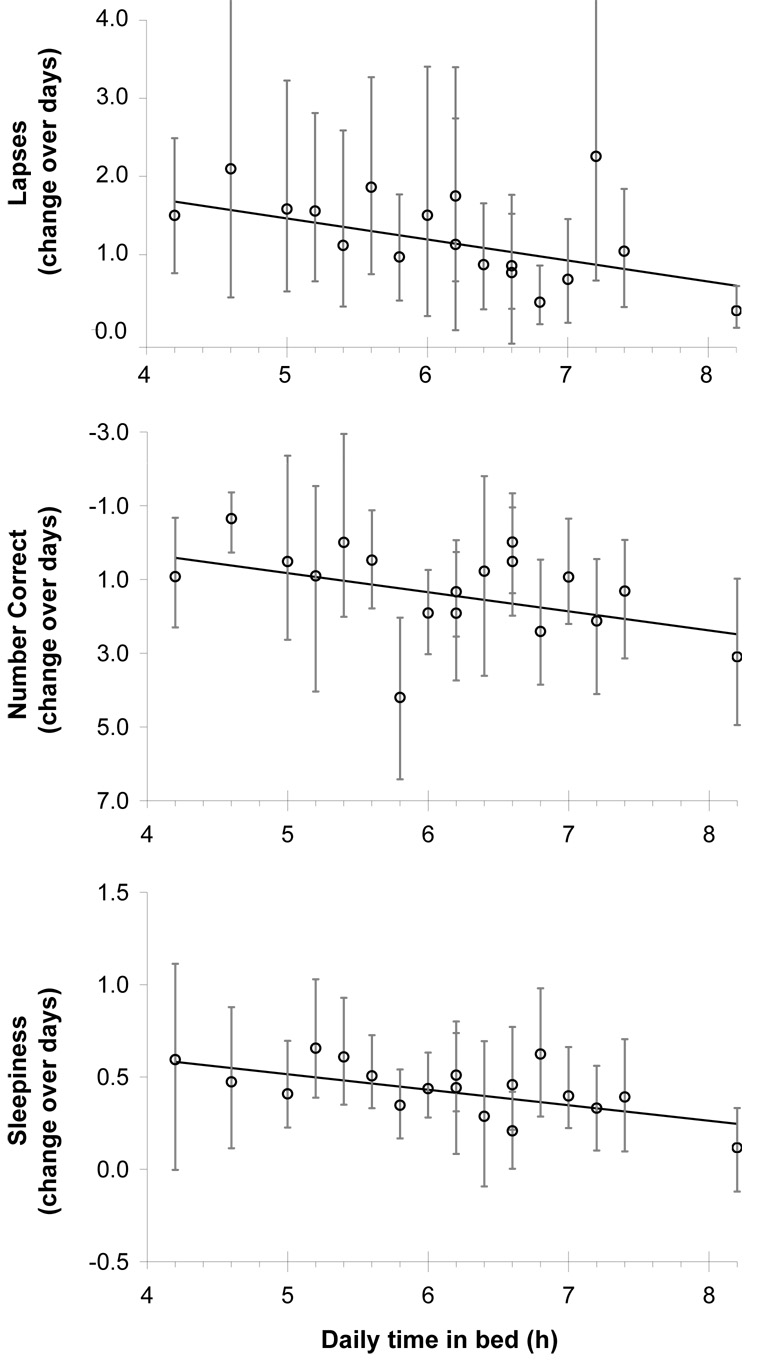
Figure 1a shows the response surface map for the rate of change of performance on the psychomotor vigilance task across sleep restriction days, as estimated for each condition specifically (β values in Eq. (1)). Subjects in the control condition (8.2 h nocturnal sleep) displayed only small, non-significant increases in lapses of attention (β=0.24, t=0.54, p=0.59). In general, the condition-specific slope increased with decreasing daily TIB (nocturnal anchor TIB plus nap TIB). Further, the rate of degradation in psychomotor vigilance task performance across the 10 sleep restriction days was found to be adequately described by a linear function of daily total TIB (Eq. (4)), with greater total TIB per 24 h steadily resulting in fewer psychomotor vigilance task lapses (χ2[1]=5.6, p=0.018)—see Figure 2a. This simpler model captured 70.1% of the variance while the more elaborate models of Eq. (1)–Eq. (3) explained no more than 70.7% of the variance.
Figure 1.
Response surface maps of neurobehavioral performance change across days (indicated on the z-axis) plotted against nocturnal anchor sleep TIB (x-axis) and diurnal nap sleep TIB (y-axis). Red dots indicate rate of change (slope) estimates for each of the 18 different conditions. Panel a. psychomotor vigilance task - lapses; Panel b. digit symbol substitution task – number of correct answers; Panel c. Stanford sleepiness scale subjective sleepiness.
Figure 2.
Neurobehavioral performance change across days as a function of daily total TIB (i.e., anchor + nap). Circles indicate estimated condition-aggregate slopes with 95% confidence intervals. The trend line marks the observed linear relationship between TIB and the slope of neurobehavioral performance over days. Panel a. psychomotor vigilance task - lapses; Panel b. digit symbol substitution task – number of correct answers; Panel c. Stanford sleepiness scale subjective sleepiness.
Thus, adding up to 16 additional model parameters to the simpler model explained only 0.6% more variance. The variance explained by the random effect over subjects for PVT performance was less than 3.2% for all models tested.
When the test bouts that were administered shortly after waking were included in the analysis, the rate of degradation in psychomotor vigilance task performance across the 10 sleep restriction days was again found to be adequately described by a linear function of daily total TIB (Eq. (4)), with greater total TIB per 24 h resulting in fewer psychomotor vigilance task lapses (χ2[1]=5.4, p=0.020).
3.2. Digit symbol substitution task performance
Figure 1b shows the response surface map for the rate of change of performance on the digit symbol substitution task across sleep restriction days, as estimated for each condition specifically (β values in Eq. (1)). Subjects in the control condition (8.2 h nocturnal sleep) displayed normal improvements in performance on the digit symbol substitution task with the expected learning curve, as indicated by daily increases in the number of correct answers (β=3.10, t=3.77, p<0.001). In general, the condition-specific slopes decreased (more impairment = fewer correct answers) with decreasing daily TIB (nocturnal TIB plus nap TIB). The rate of degradation in digit symbol substitution task performance across the 10 sleep restriction days was significantly captured by a linear function of daily total TIB (Eq. (4)), with greater total TIB per 24 h resulting in more correct answers on the digit symbol substitution task (χ2[1]=5.6, p=0.045)—see Figure 2b. This model explained 67% of the variance. However, differentiating between each anchor and nap sleep duration (Eq. (2)) resulted in significantly improved goodness-of-fit (χ2[8]=20.5, p=0.009). Yet, the additional model complexity (8 more parameters) only explained an additional 2% of the variance. Differentiating between each of the 18 different conditions separately (χ2[8]=6.2, p=0.62) provided no further significant enhancement to the goodness-of-fit and the eight additional parameters explained only 0.5% more variance. The variance explained by the random effect over subjects for DSST performance was less than 9.7% for all models tested.
When the test bouts that were administered shortly after waking were included in the analysis, the rate of degradation in digit symbol substitution task performance across the 10 sleep restriction days was again found to be best described by a model differentiating between each anchor and nap sleep duration (χ2[8]=21.5, p=0.006).
3.3. Subjective Sleepiness
Figure 1c shows the response surface map for the rate of change of subjective sleepiness across sleep restriction days, as estimated for each condition specifically (β values in Eq. (1)). Subjects in the control condition (8.2 h anchor sleep) displayed only small, non-significant daily increases in subjective sleepiness (β=0.12, t=0.99, p=0.33). In general, the condition-specific slope increased with decreasing daily TIB (nocturnal anchor TIB plus nap TIB). Further, the rate of increase of subjective sleepiness across the 10 sleep restriction days was found to be adequately described by a linear function of daily total TIB (Eq. (4)), with greater total TIB per 24 h resulting in lower subjective sleepiness ratings (χ2[1]=7.6, p=0.006)—see Figure 2c. This simpler model captured 53.6% of the variance while the more elaborate models of Eq. (1)–Eq. (3) explained no more than 55.2% of the variance. Thus, adding up to 16 additional model parameters to the simpler model explained only 1.6% more variance. The variance explained by the random effect over subjects for subjective sleepiness was less than 9.7 % for all models tested.
When the test bouts administered shortly after waking were included in the analysis, the rate of increase of subjective sleepiness across the 10 sleep restriction days was again found to be adequately described by a linear function of daily total TIB (Eq. (4)), with greater total TIB per 24 h resulting in lower ratings of subjective sleepiness (χ2[1]=7.2, p=0.007).
4. DISCUSSION
In agreement with the results of earlier dose-response studies of chronic sleep restriction [2, 3], psychomotor vigilance task performance, digit symbol substitution task performance, and subjective assessments of sleepiness were observed to be primarily a function of total time in bed per 24 h, with less total time in bed consistently resulting in greater accumulation of performance impairment and subjective sleepiness across days. For psychomotor vigilance performance and subjective sleepiness, the rate of impairment across days was found to be approximately linearly related to total time in bed per 24 h. For psychomotor vigilance, the current findings are in agreement with the results of an earlier dose-response study [2]; however the previous study did not explicitly model the relationship of rate of change to daily time in bed for subjective sleepiness. Simliar to previous studies, the present study demonstrated that the accumulation of impairment manifests in a range of critical neurobehavioral functions including decreases in psychomotor vigilance reaction speed, more frequent lapses in attention, reduced speed and accuracy of cognitive throughput, and subjective assessments of more sleepiness throughout the waking day.
An entirely new finding in the present study is that the dose-response relationships between daily sleep ration and the rate of impairment across days persisted regardless of whether the sleep was obtained in a single consolidated episode or in two split sleep episodes each day, and independent of how the amount of daily sleep was divided among nocturnal anchor sleep and diurnal nap sleep periods. This was true for the results of the psychomotor vigilance task and the Stanford sleepiness scale, and to a large extent also for the digit symbol substitution task. For the latter, however, accounting for nocturnal sleep duration and diurnal nap sleep duration explicitly provided a 2% enhancement in explained variance. Unlike the psychomotor vigilance task and the Stanford sleepiness scale, the digit symbol substitution task displays considerable learning effects [2]. As such, subtle differences among conditions in sleep architecture associated with the different placements of the sleep episodes [6] may have differentially affected the learning curve [18].
The results of this study suggest that splitting sleep up does not negatively affect daytime neurobehavioral performance compared to a consolidated sleep period of the same total duration. Since circadian phase has a profound effect on both the efficiency and structure of sleep [6], this finding must be interpreted only for conditions of the current experiment, where anchor sleeps occurred in the nocturnal portion of the circadian cycle and nap sleep in the diurnal portion. A separate experiment in which anchor and nap sleep placements are reversed in circadian time is being completed and will provide needed information on the extent to which circadian placement of split sleeps affects total sleep obtained and its recovery potential.
In a previous analysis of the present study, sleep efficiency and total sleep time were found to be a function of total time in bed per 24 h and largely independent of how sleep was divided among nocturnal sleep and diurnal nap sleep periods [9]. Moreover, the systematic relationship between total daily time in bed and waking neurobehavioral outcomes observed in this study suggests that time in bed (as a surrogate for total physiological sleep time) is substantively predictive of daytime performance. This has useful implications for space operations, as time in bed can be estimated using inexpensive, noninvasive, space flight-ready technologies such as actigraphy [19].
Taken together with the previous results on sleep efficiency, the currently reported results about neurobehavioral performance indicate that split sleep schedules offer no more recovery than consolidated sleep schedules, but also do not negatively impact recovery. Thus, split sleep schedules may be useful to enhance the flexibility of sleep/work schedules for space operations that involve restricted nocturnal sleep opportunities due to mission-critical task scheduling. This result also generalizes to all continuous industrial applications characterized sleep restriction, night operations, and shift work such as maritime operations, military operations, power generation, and manufacturing.
ACKNOWLEDGMENTS
This research was supported by the National Space Biomedical Research Institute through NASA NCC 9–58 and by National Institutes of Health grant M01-RR00040, and in part by AFOSR grant FA9550-05-1-0086 (Hans Van Dongen, Ph.D.) and the Institute for Experimental Psychiatry Research Foundation. We thank Greg Maislin for contributing the response surface modeling statistical approach and analytic techniques; Janet Mullington, James Crabbe and Scott Doran for participating in data acquisition; and Michele Carlin, Claire Fox, Adrian Ecker, John W. Powell IV, Martin Szuba and Nick Price for making the project technically feasible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mallis MM, DeRoshia CW. Circadian rhythms, sleep, and performance in space. Aviation Space and Environmental Medicine. 2005;76(6):B94–B107. [PubMed] [Google Scholar]
- 2.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 3.Belenky G, Wesensten DR, Thorne ML, Thomas HC, Sing DP, Redmond MB, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. Journal of Sleep Research. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 4.Dinges DF, Baynard M, Rogers NL. Chronic sleep restriction. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: W.B. Saunders; 2005. pp. 67–76. [Google Scholar]
- 5.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. Journal of Clinical Endocrinology and Metabolism. 2004;89(5):2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 6.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. Journal of Neuroscience. 1995;15(5 pt 1):3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. Journal of Biological Rhythms. 1999;14(6):557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 8.Achermann P, Borbély AA. Simulation of daytime vigilance by the additive interaction of a homeostatic and a circadian process. Biological Cybernetics. 1994;71:115–121. doi: 10.1007/BF00197314. [DOI] [PubMed] [Google Scholar]
- 9.Mollicone DJ, Van Dongen HPA, Dinges DF. Optimizing sleep/wake schedules in space: Sleep during chronic nocturnal sleep restriction with and without diurnal naps. Acta Astronautica. 2007;60(4–7):354–361. [Google Scholar]
- 10.de Souza L, Benedito-Silva MLN, Pires D, Poyares S, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26(1):81–85. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 11.Minors DS, Waterhouse JM. Anchor sleep as a synchronizer of rhythms on abnormal routines. International Journal of Chronobiology. 1981;7(3):165–188. [PubMed] [Google Scholar]
- 12.Minors DS, Waterhouse JM. Does Anchor Sleep Entrain Circadian- Rhythms - Evidence from Constant Routine Studies. Journal of Physiology-London. 1983 DEC;345:451–467. doi: 10.1113/jphysiol.1983.sp014988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dongen HPA, Olofsen E, Dinges DF, Maislin G. Mixed-model regression analysis and dealing with interindividual differences. Numerical Computer Methods, Pt E. 2004:139–171. doi: 10.1016/S0076-6879(04)84010-2. [DOI] [PubMed] [Google Scholar]
- 14.Van Dongen HPA, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: Importance and techniques. Aviation Space and Environmental Medicine. 2004;75(3):A147–A154. [PubMed] [Google Scholar]
- 15.Olofsen E, Dinges DF, Van Dongen HPA. Nonlinear mixed-effects modeling: Individualization and prediction. Aviation Space and Environmental Medicine. 2004;75(3):A134–A140. [PubMed] [Google Scholar]
- 16.Myers RH, Montgomery DC. Response surface methodology : process and product optimization using designed experiments. 2nd ed. New York: J. Wiley; 2002. [Google Scholar]
- 17.DiCiccio TJ, Efron B. Bootstrap confidence intervals. Statistical Science. 1996;11(3):189–212. [Google Scholar]
- 18.Walker MP, Stickgold R. It's practice, with sleep, that makes perfect: Implications of sleep-dependent learning and plasticity for skill performance. Clinics in Sports Medicine. 2005;24(2):301-+. doi: 10.1016/j.csm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Dijk DJ, Neri DF, Wyatt JK, Ronda JM, Riel E, Cecco ARD, Hughes RJ, Elliott AR, Prisk GK, West JB, Czeisler CA. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2001;281(5):R1647–R1664. doi: 10.1152/ajpregu.2001.281.5.R1647. [DOI] [PubMed] [Google Scholar]