Abstract
OBJECTIVES
To investigate if the PPARγ agonist pioglitazone modulates inflammation through PPARα mechanisms.
BACKGROUND
The thiazolidinediones (TZDs) pioglitazone and rosiglitazone are insulin-sensitizing PPARγ agonists used to treat type 2 diabetes (T2DM). Despite evidence for TZDs limiting inflammation and atherosclerosis, questions exist regarding differential responses to TZDs. In a double-blinded, placebo-controlled 16 week trial among recently diagnosed T2DM subjects (n=34), pioglitazone treated subjects manifest lower triglycerides (TG) and lacked the increase in soluble VCAM-1 (sVCAM-1) evident in the placebo group. Previously we reported PPARα but not PPARγ agonists could repress VCAM-1 expression. Since both TG-lowering and VCAM-1 repression characterize PPARα activation, we studied pioglitazone’s effects via PPARα.
METHODS
Pioglitazone effects on known PPARα responses - ligand binding domain (LBD) activation and PPARα target gene expression - were tested in vitro and in vivo, including in wildtype and PPARα-deficient cells and mice, and compared to other PPARγ (rosiglitazone) and PPARα (WY14643) agonists.
RESULTS
Pioglitazone repressed endothelial TNFα-induced VCAM-1 mRNA expression and promoter activity, and induced hepatic IκBα in a manner dependent on both pioglitazone exposure and PPARα expression. Pioglitazone also activated the PPARα LBD and induced PPARα target gene expression, with in vitro effects that were most pronounced in endothelial cells. In vivo, pioglitazone administration modulated sVCAM-1 levels and IκBα expression in wildtype but not PPARα-deficient mice.
CONCLUSIONS
Pioglitazone regulates inflammatory target genes in hepatic (IκBα) and endothelial (VCAM-1) settings in a PPARα-dependent manner. This data offers novel mechanisms that may underlie distinct TZD responses.
Keywords: Inflammation, VCAM-1, PPARs
INTRODUCTION
The increased risk for atherosclerotic complications evident in individuals with type 2 diabetes mellitus (T2DM) has driven interest in the cardiovascular effects of anti-diabetic therapies both in use and under development (1,2). The number of insulin resistance-associated abnormalities that also promote atherosclerosis focused attention on the cardiovascular effects of insulin sensitizing agents (3). In this context, thiazolidinediones (TZDs) held significant promise as insulin sensitizers that lower glucose and reportedly limited atherosclerosis and inflammation in vitro and in vivo in both mice and humans (4). Recently, the TZDs pioglitazone and rosiglitazone have been scrutinized for their possible distinct effects, including those on the cardiovascular system (5).
TZDs bind to and activate PPARγ, a ligand-activated transcription factor that regulates key metabolic pathways, including adipogenesis and insulin sensitivity (6,7). Pioglitazone and rosiglitazone are approved as “highly selective” PPARγ agonists (8). PPARγ is also expressed in vascular and inflammatory cells where its activation can regulate target genes relevant to atherosclerosis (9). Although pioglitazone and rosiglitazone target the same PPAR isoform, (4), recent human TZD data has raised the possibility of variable responses between these agents and as well as with other PPARγ agonists. In several studies, including one head-to-head clinical trial, pioglitazone and rosiglitazone have had variable as well as divergent effects on triglycerides (10,11). Meta-analysis data has suggested possible adverse cardiovascular outcomes with rosiglitazone (12–14), although not without controversy (15,16); similar studies with pioglitazone have not shown cardiovascular safety signals, including one prospective clinical trial (13, 54). Novel dual PPARα/γ agonists in development have been abandoned for various adverse effects, including cardiovascular responses (12), raising concerns about dual PPARα/γ therapeutics (17,18). Clearly additional mechanistic insight into how specific PPAR agonists exert their effects is needed.
During a small 16 week study on inflammatory markers in patients with recently diagnosed T2DM randomized to pioglitazone or placebo, we noted that levels of soluble vascular cell adhesion molecule-1 (VCAM-1), an early player in atherogenesis, increased in the placebo group but not those on pioglitazone (19). Previously we reported that PPARγ activation had no effect on VCAM-1 expression in vitro, although those studies did not include piogitazone (20). In contrast, PPARα agonists decrease VCAM-1 in a PPARα-dependent manner (20,21). Although limited in nature, this clinical data raised the hypothesis that pioglitazone might repress inflammation, including VCAM-1 expression, in part via PPARα. Although previously raised as a possibility, we studied pioglitazone effects on known PPARα responses in more definitive models, including PPARα-deficient cells and mice (22). We provide here the first evidence that pioglitazone represses key endothelial and hepatic inflammatory responses in vitro and in vivo in mice in a PPARα-dependent manner.
METHODS
Human studies
Subjects
Individuals meeting the American Diabetes Association criteria for T2DM - fasting plasma glucose (FPG) ≥ 126 mg/dL with a second confirmatory measurement or a 2 hour plasma glucose ≥ 200 mg/dL during oral glucose tolerance testing (2 h-OGTT) – were enrolled in a randomized, prospective, double-blinded, placebo-controlled clinical trial (n = 34). The study cohort included subjects with newly diagnosed T2DM or confirmed T2DM on a non-pharmacological dietary intervention for at least 4 weeks prior to the first study visit; all subjects were either drug-naïve or off any anti-diabetic medication for at least 4 weeks. Exclusion criteria included prior TZD or insulin treatment, hemoglobin A1c (HbA1c) ≥ 10%; FPG ≥ 260 mg/dL; history of myocardial infarction, unstable angina, cerebral vascular accident, transient ischemic attack, coronary artery bypass graft (CABG) or percutaneous transluminal coronary angioplasty; NYHA class III or IV congestive heart failure; diastolic blood pressure > 100 mmHg and/or systolic blood pressure > 160 mmHg; total cholesterol > 300 mg/dL and/or triglycerides (TG) > 600 mg/dL; serum creatinine >1.5 mg/dL; current use of systemic corticosteroids, immunosuppressants or androgens; any severe acute or chronic disease; other medical condition possibly interfering with study participation or assessment of the trial investigational products.
Protocol
Participants, recruited from the Joslin Diabetes Center outpatient clinics or local media advertisement, were randomized 1:1 to either pioglitazone 30 mg or a matching placebo once a day for the first 4 weeks, with a subsequent increase to pioglitazone 45 mg or matched placebo once a day for the next 12 weeks (total 16 week intervention period). Both groups were evaluated at baseline and study conclusion. Patients were instructed on an isocaloric diet (50% carbohydrates, 20% protein, 30% fat) and to maintain their usual physical activity.
Laboratory evaluations
All measurements were performed at the Clinical Research Center, Joslin Diabetes Center (23). Plasma sVCAM-1 concentrations in mice and humans were determined in duplicate blinded samples using ELISA (Quantikine, R&D Systems). Intra-assay variation was less than 10%; the sVCAM-1 detection limit was 0.6 ng/mL. To analyze sVCAM-1 responses in different subgroups, national guideline cutpoint values were used: above and below FPG 126 mg/dL, 2h-OGTT 200 mg/dL, HbA1c 7%, TG 150 mg/dL, LDL-cholesterol (LDL-C) 100 mg/dL, HDL-C 40 mg/dL (24,25).
Reagents
Pioglitazone hydrochloride (Pioglitazone HCl) was a gift from Takeda Pharmaceuticals North America (Lincolnshire, IL); WY14643 (Biomol, Plymouth Meeting, PA); rosiglitazone (BRL49653, GlaxoSmithkline, Research Triangle Park, NC). All media (BioWhittaker, Walkersville, MD), contained fungizone, penicillin, streptomycin and plasma as indicated. Human and murine TNFα were purchased from R&D Systems (Minneapolis, MN); Escherichia coli O111:B4 lipopolysacharide (LPS) and 2, 2, 2-tribromoethanol (Sigma-Aldrich, St. Louis, MO).
Cell Culture
Human ECs isolated from saphenous veins (HSVEC) were cultured in M199 medium, endothelial cell growth factor (ECGF), 5% fetal calf serum (20). Bovine Aortic Endothelial Cells (BAEC) were grown in Dulbecco modified Eagle medium (DMEM, 10% fetal bovine serum, glutamine, penicillin, streptomycin and fungizone) (26). TNFα stimulations were done at 10 ng/ml. PPARα+/+ (129S1/SvImJ) mice were obtained from Jackson Laboratories (Bar Harbor, ME). PPARα−/− mice (129S4/SvJae) were a generous gift from F. Gonzalez (National Institute of Health). Murine ECs from 1-month-old PPARα+/+ and PPARα−/− mouse hearts were isolated using double selection with intercellular adhesion molecule 2 and platelet endothelial cell adhesion molecule 1 antibodies (BD PharMingen) bound to Dynabeads (Dynal, Lake Success, NY) as before (27).
Plasmids
Human GAL4-PPARα- or PPARγ- ligand binding domain (LBD) constructs (pSG5 vector, S. Kliewer, University of Texas Southwestern, Dallas, TX) were used for trans-activation assays. The VCAM-1 promoter construct (755 upstream bp, T. Collins, Children’s Hospital, Boston, MA) contains the major regulatory elements (AP-1, NF-κB, PPAR) (20). cDNA probes for Northern blotting included human VCAM-1 (2.1kb Kpn/Sphl fragment, G. Garcia-Cardena, Brigham and Women’s Hospital, Boston, MA); mouse full-length PPARα (2kb cDNA fragment); human acyl-CoA-oxidase (ACO; ATCC, Manassas, VA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, ATCC).
Transient transfection assays
Standard GAL4-PPAR-LBD assays were performed as before (24-well plates, 2.5×104 BAECs/well, passage 2–5, FuGENE 6 (Roche Diagnostics, Indianapolis, IN) (26) in DMEM (1% delipidated fetal calf serum) using human GAL4-PPARα or γ-LBD, pUASx4-TK-luc, and β-galactosidase (pcDNA-β-Gal) constructs before stimulation (24h later) with the compounds indicated for 16h. For VCAM-1 promoter studies, BAECs were plated in 1% Nutridoma SP (Roche Diagnostics), transfected with the VCAM-1 promoter construct (24h), and then pre-treated with pioglitazone (3 – 30 µM) or WY14643 (25 – 225 µM, 3h) before TNFα stimulation (12 h). Responses were normalized to co-transfected β-Galactosidase (pcDNA3) activity using chlorophenol red-β-D-galactopyranoside substrate (Roche Diagnostics) as before (26). For PPARα reconstitution experiments, murine PPARα−/− ECs were plated in 1% delipidated fetal calf serum before transfection with either PPARα (mouse full-length PPARα-pSG5) or empty vector (pSG5, 24 h). Cells were pre-treated with pioglitazone (10 µM) or WY14643 (100 µM) for 18 h before mouse TNFα (10 h) stimulation as indicated.
RNA Extraction and Northern Blot Analysis
Total RNA was isolated using RNeasy (Qiagen, Valencia, CA) before gel separation and transfer (Hybond-N, Amersham Pharmacia, Piscataway, NJ). Northern and Western blots were quantitated using densitometry (Epson Scan 1200, Image-Pro Plus 5.1).
Western Blotting
Standard Western blot analysis of human EC lysates were performed using rabbit polyclonal antibody against human IκBα (1:500, Santa Cruz Biotechnology, Santa Cruz, CA) and monoclonal antibody against GAPDH (1:10,000, Biodesign, Saco, ME). For in vivo studies, frozen livers were pulverized, added to RIPA buffer (50 mM Tris, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) with freshly added protease inhibitors, centrifuged (10’, 13,000 rpm, 4°C), and the protein extract boiled in electrophoresis buffer before gel separation (15% polyacrylamide, β-mercaptoethanol-reducing conditions) and transfer (Immobilon-P membranes, semi-dry transfer, 1h, 16V). After blocking (5% delipidated milk, 20 mM Tris, 55 mM NaCl, 0.1% Tween 20, 1 h), immunoblotting with the IκBα and GAPDH antibodies described above was performed using chemiluminescence (PerkinElmer Life Sciences, Boston, MA).
Animal studies
3 month-old age- and sex-matched PPARα+/+ and PPARα−/− mice were divided into two feeding groups (n = 9/genotype). Group One received pioglitazone (20 mg/kg body weight in 0.5% w/v methylcellulose) by gavage once daily for seven days. Group Two was treated similarly with vehicle (0.5% w/v methylcellulose). Mice received free access to water, ordinary laboratory diet and standard animal care (Harvard University guidelines). On Day One, mice received a survival dose of anesthetic (2, 2, 2 – tribromoethanol, 250 mg/kg body weight) intraperitoneally before retroorbital blood draw for baseline serum measurements. On Day Eight, retroorbital blood draws were repeated on a random subgroup of mice (n = 5). The next day, all mice received LPS (12 mg/kg body weight) intraperitoneally; four hours later, mice were anesthetized and blood drawn by vena cava puncture and allowed to clot overnight (4°C). Mice were then sacrificed and livers removed immediately, rinsed (0.9% NaCl) and snap-frozen for further analysis. Blood samples were centrifuged (2000 × g, 4°C, 20’) and serum sVCAM-1 concentrations determined in duplicate as described above.
Statistical Analysis
Statistical analysis, performed in conjunction with the Brigham and Women’s Hospital Center for Clinical Research Biostatistics Core Laboratory, employed Statistical Package for Social Sciences (SPSS, v.16.0), SAS 9.1, and Analyze-it for Microsoft Excel (v1.71). Results are presented as mean ± SD or mean ± SE. Means for baseline clinical characteristics of the human study participants were compared using the independent-Student’s t test. For within-group analysis (the baseline study vs. the follow-up assessment), two-sided paired Student’s t test for parametric data was used. For all other among-group comparisons, the Mann-Whitney test was used. Pearson correlation coefficients were calculated to test the association between variables. p≤0.05 was regarded as significant.
RESULTS
Changes in sVCAM-1 on pioglitazone vs placebo in recently diagnosed T2DM subjects
Pioglitazone (n = 19) and placebo (n = 15) groups were similarly matched on all baseline variables, including sVCAM-1 levels (Table). Pioglitazone significantly improved FPG (162.2 ± 13.6 vs. 125.4 ± 7.1 mg/dL, p = 0.002), 2h-OGTT (273.5 ± 19 vs. 216.3 ± 12.6, p =0.001), TG (160.7 ± 24.9 vs. 129.1 ± 11.4, p=0.008), and TG/HDL ratio (3.5 ± 0.5 vs. 3.1 ± 0.2, p = 0.02), all as compared to placebo at baseline versus study end (Table).
Table 1.
Characteristics of the diabetic patients at baseline and after 16 weeks of intervention with placebo or pioglitazone.
| Placebo (n=15) |
Pioglitazone (n=19) |
|||||
|---|---|---|---|---|---|---|
| baseline | 16 weeks | p value | baseline | 16 weeks | p value | |
| Age, years | 58.8±11 | 61.3±5.9 | ||||
| Men/Women | 9/6 | 16/3 | ||||
| BMI, kg/m2 | 31.6±3.1 | 31.09±3 | ns | 31.9±3.7 | 32.1±3.5 | ns |
| Weight, kg | 94.1±9.7 | 93.3±9.1 | ns | 95.8±13.7 | 96.6±12 | ns |
| Waist, cm | 108.8±7.8 | 108.8±9.7 | ||||
| SBP, mmHg | 125.5±15.7 | 129.3±13.3 | ||||
| DBP, mmHg | 81.7±9.7 | 77.4±8.7 | ||||
| FPG, mg/dL | 146.4±10.3 | 140.9±7.7 | ns | 162.2±13.6 | 125.4±7.1 | 0.002 |
| 2h-OGTT, mg/dL | 263.3±13.8 | 265.6±15.2 | ns | 273.5±19 | 216.3±12.6 | 0.001 |
| Total-C, mg/dL | 197.4±9.6 | 177.3±10.9 | ns | 203.8±9.9 | 169.2±7.4 | ns |
| LDL-C, mg/dL | 119±9.1 | 88.4±7.5 | ns | 128.4±8.3 | 103.1±7 | ns |
| HDL-C, mg/dL | 47±1.7 | 35±1.9 | 0.001 | 45.9±2.1 | 41.4±2.1 | ns |
| TG, mg/dL | 169.8±15.3 | 150.3±16.1 | ns | 160.7±24.9 | 129.1±11.4 | 0.008 |
| TG/HDL ratio | 3.5±0.3 | 4.6±0.6 | ns | 3.5±0.5 | 3.1±0.2 | 0.02 |
| sVCAM-1, ng/mL | 512.1±45.7 | 600.5±41.7 | 0.008 | 470.4±33.9 | 486.7±45.7 | ns |
| hsCRP, mg/L | 1.3† | 1.3† | ns* | 2.6† | 0.8† | ns* |
| TNFα, ng/mL | (0.44–3.89) 1.5±0.09 |
(0.49–89.3) 1.8±0.1 |
ns | (0.25–23.8) 1.3±0.08 |
(0.17–8.75) 1.2±0.08 |
ns |
Data are showed as mean SE with 95% confidence interval (CI) or median (†). No baseline differences in any variables (independent Student’s t test); p<0.05 shows significant differences within group (paired-Student’s t test)
Median test (*) between groups pre or post treatment. BMI (Body Mass Index); SBP (Systolic Blood Pressure); DBP (Diastolic Blood Pressure); FPG (Fasting Plasma Glucose); OGTT (Oral Glucose Tolerance Test), ns = non-significant.
During the study, sVCAM-1 levels rose significantly in patients with recently diagnosed T2DM randomized to placebo alone (baseline 512.1 ± 45.7 ng/mL vs. study conclusion 600.5 ± 41.7 ng/mL, p<0.008, within group analysis, Table). In contrast, sVCAM-1 levels did not rise among pioglitazone-treated subjects (baseline 470.4 ± 32.3 vs. conclusion 486.7 ± 43.3 ng/mL, ns, within group analysis, Table). Using a mixed design linear regression model to control for baseline levels of multiple parameters, only age had a significantly impact on sVCAM-1 levels (See Supplementary Table A). After controlling for age, sVCAM-1 levels differed significantly between placebo and pioglitazone groups (p=0.03, Supplementary Table B). TNFα levels also increased over time from 1.5±0.09 to 1.8±0.1 ng/mL in the placebo group but decreased from 1.3±0.08 to 1.2±0.08 ng/mL in the pioglitazone groupalthough not in a statistically significant way. Baseline levels of hs-CRP and sVCAM-1 were also significantly correlated (r=0.45, p = 0.02).
To generate hypotheses as to biologic mechanisms underlying possible pioglitazone effects on repressing the sVCAM-1 increase seen in patients on placebo, responses were analyzed according to intervention arm and subgroups stratified by accepted TG, HDL-C, LDL-C, FPG, and HbA1c cutpoints (see Methods). Only TG subgroups revealed differences in sVCAM-1 levels. Using the National Cholesterol Education Program TG cutpoint of 150 mg/dL (28), significant sVCAM-1 increases were restricted to those with higher baseline TG levels (≥150 mg/dL, n = 9; from baseline 506 ± 63.9 ng/mL to 683.1 ± 56.4 ng/mL, p<0.03); sVCAM-1 levels did not differ significantly in placebo-treated subjects with lower baseline TG (<150 mg/dL, n = 6). Among pioglitazone-treated subjects, sVCAM-1 levels did not differ in either higher or lower TG subgroups (data not shown).
Pioglitazone represses TNFα-induced VCAM-1 mRNA expression in endothelial cells
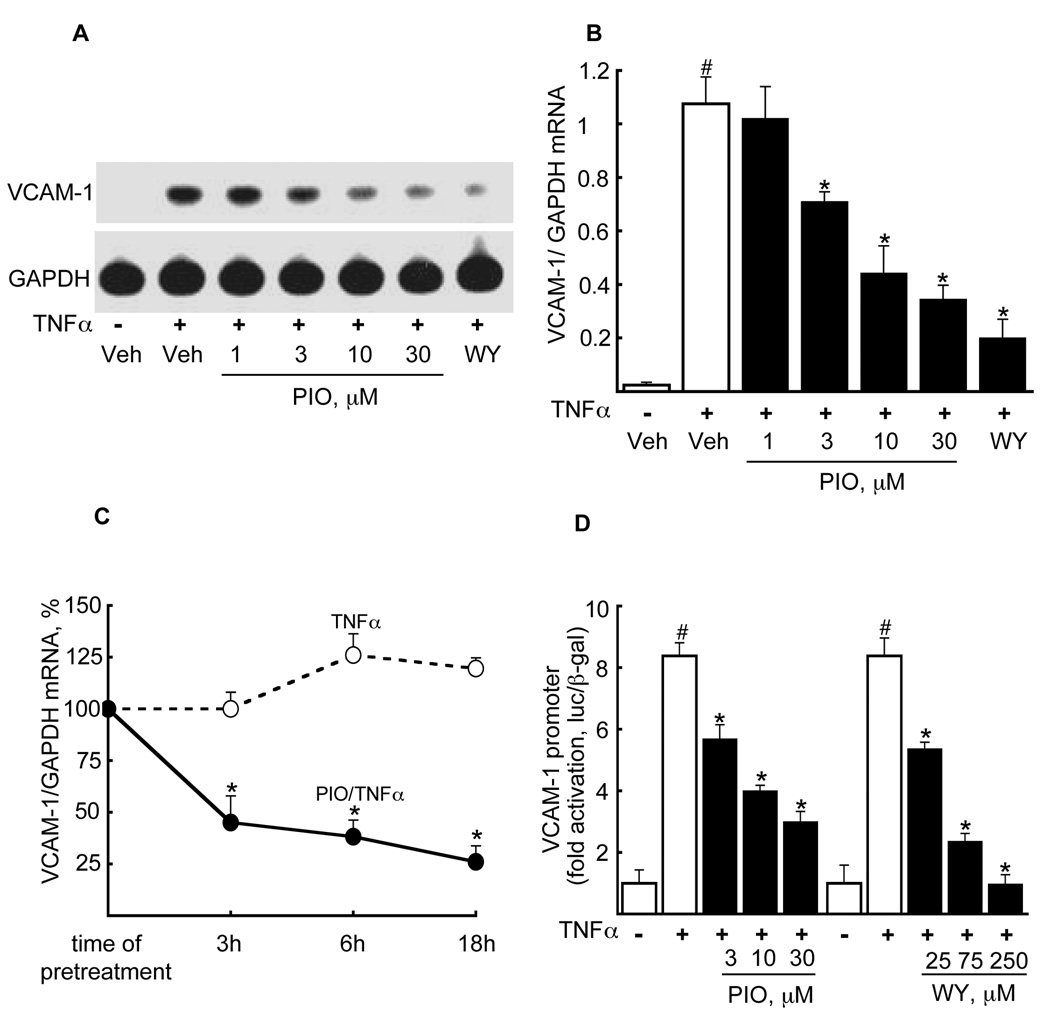
Given the data above, we tested pioglitazone’s effects on TNFα-induced VCAM-1 mRNA expression in HSVECs (18 h pre-treatment) using a concentration range commonly used in vascular biology studies and that overlaps pioglitazone levels reported in humans (29). Pioglitazone inhibited VCAM-1 mRNA induction in a dose-dependent manner (Fig. 1A). For comparison, the known repression of VCAM-1 mRNA by the PPARα agonist WY14643 (100 µM) is also shown (Fig. 1A). Quantification of relative changes in VCAM-1/GAPDH mRNA expression using densitometry reveals a significant pioglitazone effect at the concentrations shown (Fig. 1B, 3–30 µM, p<0.05 for each). As previously reported, rosiglitazone (BRL49653, BRL) had no significant effect on VCAM-1 expression (data not shown) (20,30–32). Pioglitazone-mediated repression of VCAM-1 expression also varied as a function of pioglitazone exposure (3, 6, 18 h; 10 µM) prior to TNFα stimulation (maximal 74% reduction at 18 h, p<0.05, Fig. 1C).
Figure 1. Pioglitazone reduces TNFα-induced VCAM-1 mRNA expression in a dose- and time-dependent manner in human saphenous vein ECs (HSVECs).
(A) Northern blot analysis of TNFα-induced VCAM-1 mRNA expression was performed on HSVECs pretreated in the absence or presence of pioglitazone (18 h) at the concentrations shown prior to TNFα stimulation (10 ng/mL, 10 h). The effects of the PPARα agonist WY14643 (100 µM) are provided for comparison. One representative Northern blot (n=3) is shown. (B). The effect of the pioglitazone concentrations on VCAM-1/GAPDH mRNA was quantified from the Northern blots above. (n=3, #p<0.05, TNFα-induced vs. vehicle, *p<0.05, pioglitazone/TNFα vs. TNFα alone, Mann-Whitney test). (C) The time dependent effects of pioglitazone exposure (10 µM) on TNFα-induced VCAM-1 expression was tested in HSVECs using Northern blotting. Results are shown as a percent of the TNFα effect alone at 3 h, mean ± SD (n = 3; *, p<0.05). (D) The effect of pioglitazone versus vehicle on the human VCAM-1 promoter transiently transfected into BAEC before TNFα stimulation are shown (left). For comparison, the effect of the PPARα agonist WY14643 on the VCAM-1 promoter is also shown (right). All responses were normalized to β-galactosidase (pCMV-β-Gal) (n = 3 per each treatment, #p<0.05 TNFα vs. vehicle; *p<0.05 pioglitazone or WY14643 vs. TNFα alone, Mann-Whitney test).
We next considered if pioglitazone could inhibit human VCAM-1 promoter activity, as reported for synthetic PPARα agonists (20). A human VCAM-1 promoter-luciferase construct was transiently transfected into bovine aortic ECs (BAECs) before testing pioglitazone (3–30 µM) effects on TNFα-induced VCAM-1 promoter-driven luciferase activity. As expected, TNFα stimulation significantly induced VCAM-1 promoter activity (8.37 ± 0.58 fold, p<0.05, Fig. 1D). Pioglitazone repressed TNFα-induced VCAM-1 promoter activity across a dose range (p<0.05, Fig. 1D); responses to the PPARα agonist WY14643 are shown for comparison (Fig. 1D).
Pioglitazone regulation of VCAM-1 mRNA expression in the genetic presence or absence of PPARα
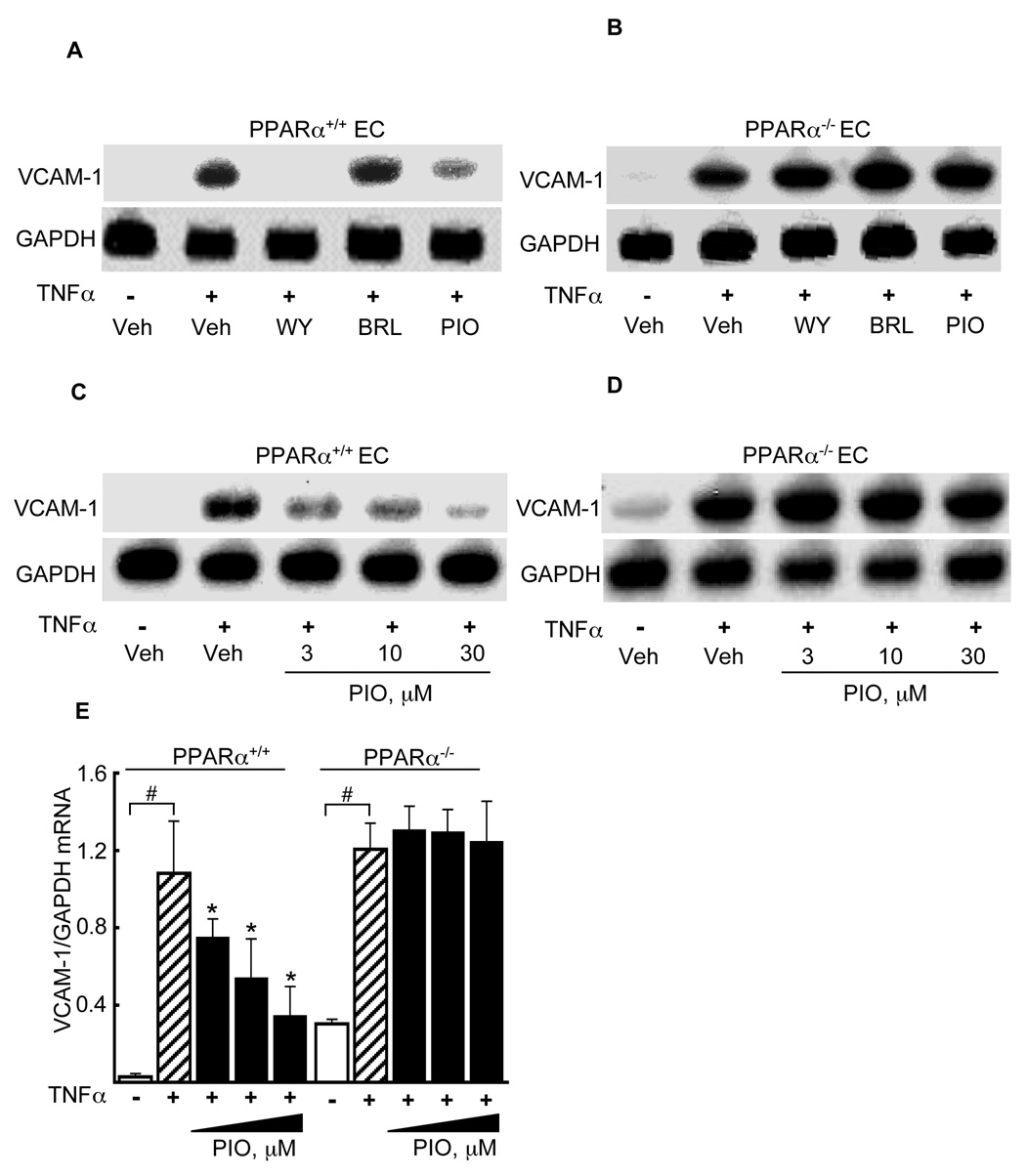
We next considered if pioglitazone’s effects on VCAM-1 required the genetic presence of PPARα. In Northen blots of microvascular ECs isolated from either wildtype (PPARα+/+) or PPARα-deficient (PPARα−/−) mouse hearts, WY14643 (100 µM) and pioglitazone (10 µM) pretreatment decreased VCAM-1 mRNA expression in PPARα+/+ but not in PPARα−/− ECs while BRL (1 µM) had no effect in either PPARα+/+ or PPARα−/− ECs (Fig 2A, B), as seen in Northern blotting. Pioglitazone significantly decreased TNFα-induced VCAM-1 mRNA expression in a dose-dependent manner (3 – 30 µM, 18 h) in wildtype EC (as compared to TNFα stimulation alone, p<0.05, Fig. 2C) but not in PPARα−/− ECs (Fig. 2D). Quantification of these responses (n=3) demonstrated a significant VCAM-1 effect at each pioglitazone dose tested in wild-type but not PPARα deficient EC (p<0.05, Fig. 2E).
Figure 2. Pioglitazone represses TNFα-induced VCAM-1 expression in a PPARα-dependent manner.
ECs isolated from PPARα+/+ (A) and PPARα−/− (B) mouse hearts were pretreated with WY14643, rosiglitazone (BRL), or pioglitazone at the concentrations shown (18 h) before mouse TNFα stimulation and subsequent Northern blotting for VCAM-1 mRNA and GAPDH expression. One representative blot of three is shown. Northern blotting for VCAM-1 expression was repeated in the presence of the dose range of pioglitazone shown in EC from PPARα+/+ (C) and PPARα−/− (D) mice. (E) Quantification of the effects of pioglitazone on VCAM-1 mRNA in PPARα+/+ and PPARα−/− EC relative to GAPDH mRNA expression (n = 3, #p<0.05 TNFα vs. vehicle; *p<0.05 pioglitazone/TNFα vs. TNFα alone, Mann-Whitney test).
Reconstitution of PPARα expression in PPARα-deficient endothelial cells
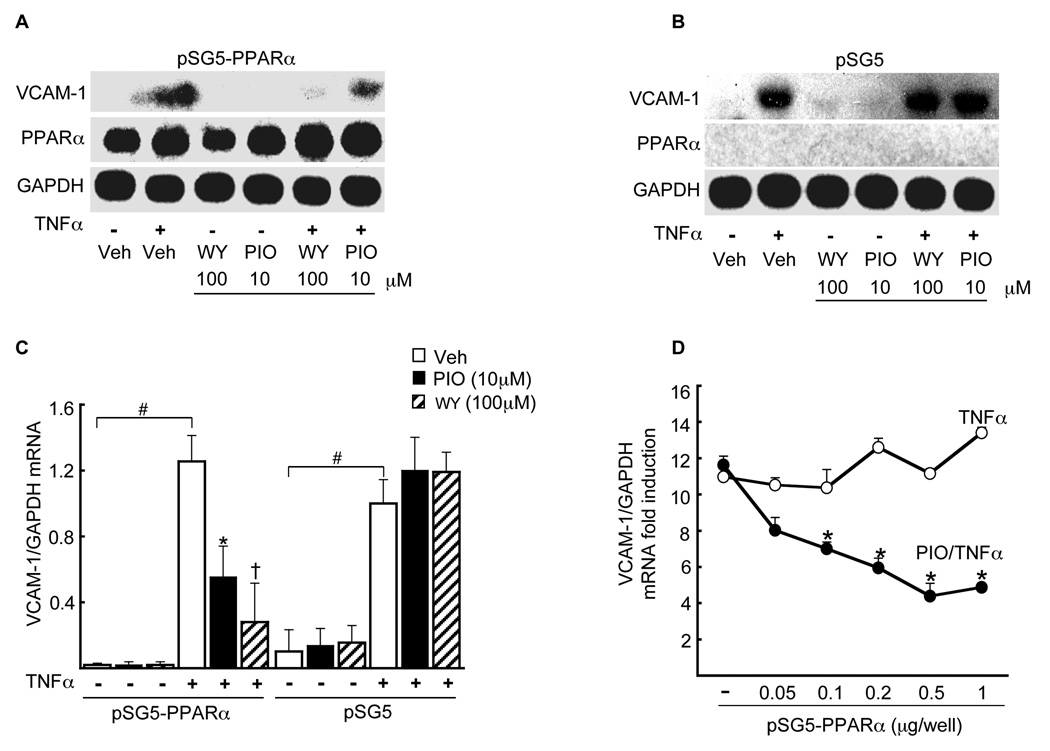
To test if reconstituting PPARα expression in PPARα-deficient ECs was sufficient to restore pioglitazone-mediated repression of VCAM-1 expression, PPARα−/− ECs were transiently transfected (24 h) with a full-length mouse PPARα cDNA (pSG5 expression vector) and compared to cells transfected with the pSG5 vector alone before pretreatment with pioglitazone (10 µM, 18 h) or WY14643 (100 µM, 18 h) and TNFα stimulation. Expressing PPARα in PPARα−/− ECs restored significant pioglitazone-induced repression of cytokine-induced VCAM-1 expression (Fig. 3A) while transfection of the pSG5 vector alone into PPARα−/− ECs had no effect on pioglitazone responses (Fig. 3B), as evident on densitometry (p < 0.05, Fig. 2C). These results indicate that PPARα expression is necessary for pioglitazone-mediated repression of TNFα-induced endothelial VCAM-1 mRNA expression.
Figure 3. PPARα is required for pioglitazone-mediated repression of TNFα induced endothelial VCAM-1 mRNA expression.
Northern blot analysis was performed on total RNA isolated from PPARα−/− ECs transfected either with PPARα-containing pSG5 overexpression vector (A) or pSG5 alone (B) before stimulation with TNFα in either the absence or presence of WY (100 µM) or pioglitazone (10 µM), with subsequent probing for VCAM-1 or GAPDH expression. (C) Quantification of the VCAM-1 mRNA response to pioglitazone relative to GAPDH mRNA expression levels (n = 3, #p<0.05 TNFα vs. vehicle; *p<0.05 pioglitazone/TNFα vs. TNFα alone; †p<0.05 WY14643/TNFα vs. TNFα alone, Mann-Whitney test). (D) Cells were transfected in (A, B) but with a concentration gradiant of pSG5-PPARα as shown before TNFα stimulation in either the absence or presence of pioglitazone 10 µM (n = 3, *p<0.05 pioglitazone/TNFα vs. TNFα alone).
Pioglitazone effects on expression of canonical PPARα-regulated target genes
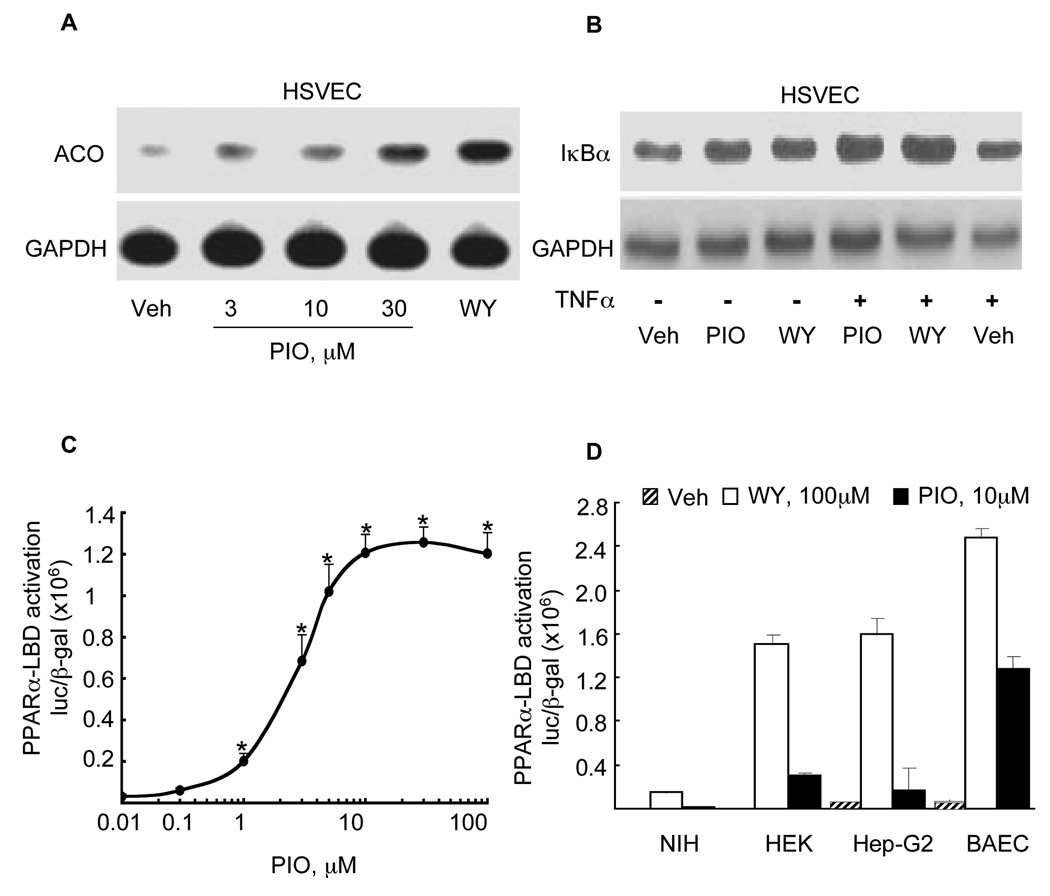
We next asked if pioglitazone also regulated expression in ECs of two other well-established PPARα-regulated targets: acyl-CoA oxidase (ACO) and IκBα. ACO contains a defined PPARα response element in its upstream promoter region (33). Pioglitazone (3–30 µM) and the PPARα agonist WY14643 (100 µM, 6 h) significantly increased ACO mRNA expression compared to untreated HSVECs (Fig. 4A). Prior reports indicate that PPARα activation increases expression of IκBα, a key regulator of inflammation (34). HSVECs were pretreated with pioglitazone (30 µM) or WY (100 µM, 16 h) either alone or before TNFα stimulation prior to Western blotting. Both WY14643 and pioglitazone increased IκBα protein levels in HSVEC (Fig. 4B). TNFα stimulation further increased the IκBα response to pioglitazone, as previously reported for PPARα agonists (Fig. 4B) (34).
Figure 4. Pioglitazone induces known PPARα target gene expression and PPARα–LBD activation in ECs.
(A) Northern blot analysis in HSVECs was performed for the PPARα target gene acyl-CoA-oxidase (ACO) and compared to GAPDH in HSVEC pretreated (16 h) with pioglitazone or WY14643 at the concentrations shown before TNFα stimulation. B) Western blot analysis for IκBα expression was performed on total protein extracts (50µg) from HSVECs treated with either pioglitazone (10 µM) or WY14643 (250 µM) before stimulation with human TNFα. (C) Standard LBD activation assays were performed in BAECs stimulated with pioglitazone at the concentrations shown (0.01–100 µM). (D) PPARα-LBD assays were done as before but comparing responses in NIH/3T3 (fibroblasts), HEK293 (human kidney epithelial), Hep-G2 (hepatic) and BAEC cell lines before stimulation with pioglitazone or WY14643 (both 10 µM). Values are expressed as luciferase/β-Gal activity mean± SD (n = 3. *p<0.05 BAEC vs. NIH/3T3, ** vs. HEK293, *** vs. Hep-G2, both Student’s t and Mann-Whitney tests).
Pioglitazone’s dependency on PPARα for VCAM-1 repression and its induction of PPARα-regulated target genes suggests that pioglitazone or one of its metabolites might activate PPARα. Prior studies considering this issue have varied considerably, with experiments in multiple cell types using PPAR-LBDs from different species (35). Standard Gal4-LBD transfection assays were performed using human PPARα-LBD transfected into BAECs before pioglitazone (0.01–100 µM) stimulation. In BAECs, pioglitazone activated the PPARα-LBD significantly and in a dose-dependent manner (1–100 µM Fig. 4C). These effects were significant although less than the PPARγ activation seen with pioglitazone; rosiglitazone had no effects on PPARα activation (data not shown). Given cell type contributions to variable PPAR-LBD responses previously reported, we compared pioglitazone effects on human PPARα-LBD assays in NIH/3T3 (fibroblast), HEK293 (epithelial), and Hep-G2 (hepatic) cell lines using either pioglitazone or WY14643 (both 10 µM), normalizing responses to β-galactosidase (pcDNA-βGal) activity. PPARα-LBD activation by either WY14643 or pioglitazone varied significantly according to cell type (Fig. 4D). Interestingly, pioglitazone’s PPARα-LBD effects were most potent (relative to WY14643) in bovine ECs (52%) compared with all other non-EC cell lines tested: 17% in NIH/3T3 17%, HEK293 21%, and Hep-G2 11% (all p<0.05, Fig. 4D). Thus PPAR responses to pioglitazone may vary depending on cell type.
Pioglitazone’s PPARα-dependent effects on hepatic IκBα protein levels in vivo
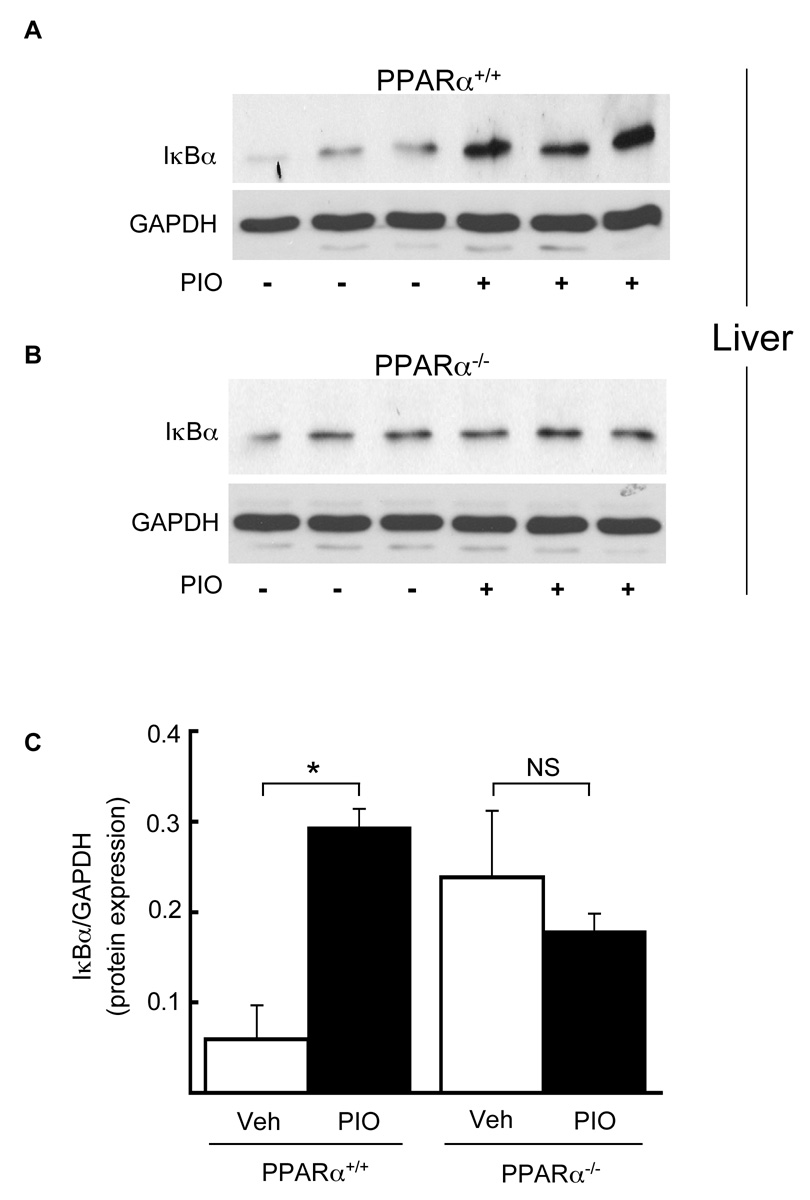
We next considered if pioglitazone regulated PPARα responses in a PPARα-dependent manner in vivo. Given the TG and sVCAM-1 effects shown here (Table), we focused on pioglitazone responses in tissues related to lipid metabolism (liver) and inflammation (endothelium), and relevant PPARα-regulated inflammatory target genes in those settings, namely IκBα and VCAM-1. PPARα+/+ (n = 4) and PPARα−/− (n = 4) mice were treated with pioglitazone (gavage, 20mg/kg body weight, 7 days) before harvesting livers and performing IκBα western blotting. Consistent with our in vitro results, pioglitazone significantly increased hepatic IκBα protein expression in PPARα+/+ (Fig. 5A) but not PPARα−/− (Fig. 5B) mice, as evident on densitometry (p < 0.05, Fig. 5C,)
Figure 5. Pioglitazone induces IκBα protein expression in vivo in a PPARα dependent manner.
PPARα+/+ (A) and PPARα−/− (B) mice were treated with pioglitazone (20mg/kg, 7 days via gavage) before livers were harvested and total protein extracted for Western blot analysis of IκBα and GAPDH protein levels. Each lane represents a single mouse. (C) The effects of pioglitazone (black bars) and vehicle (white bars) on IκBα protein expression were quantified and normalized to GAPDH expression. Mean values SD are shown (n = 4 mice/group. *p<0.05 pioglitazone vs. vehicle, Mann-Whitney test).
Pioglitazone’s PPARα-dependent effect on sVCAM-1 levels in vivo
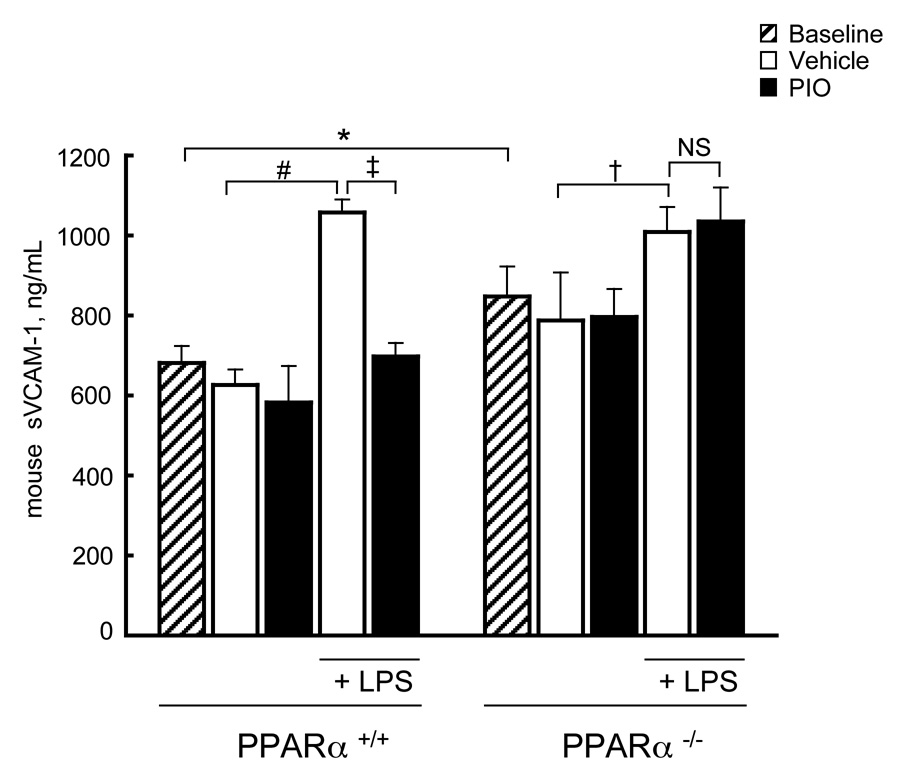
We next tested if pioglitazone repressed sVCAM-1 levels in mice in vivo in a PPARα-dependent manner. PPARα+/+ and PPARα−/− mice (9 mice/genotype/treatment) were treated (daily gavage, 7 days) with either pioglitazone (20 mg/kg body weight, 0.5% w/v methylcellulose, Group One) or vehicle alone (Group Two) before establishing baseline sVCAM-1 levels followed by LPS intraperitoneal stimulation and blood draws. Basal sVCAM-1 levels were significantly higher in PPARα−/− mice (847.4 ± 75.1 ng/mL, n = 18) versus PPARα+/+ mice (680.8± 42.4 ng/mL, n = 18), p<0.007 (Fig. 6). As expected, LPS treatment increased sVCAM-1 levels significantly in vehicle-treated PPARα+/+ mice (1058.11 ± 32.15 ng/mL, n=9, p<0.002). In contrast, LPS-induced sVCAM-1 levels in pioglitazone-treated PPARα+/+ mice were unchanged from basal levels (697.55 ± 33.78 ng/mL, n=9, p<0.01, vs LPS alone, n=9, Fig. 6). In PPARα−/− mice, pioglitazone had no effect on LPS-induced sVCAM-1 protein levels (pioglitazone, 1034.8 ± 84.8 ng/mL vs. vehicle, 1008.5 ± 62.3 ng/mL, n = 9, Fig. 6).
Figure 6. Pioglitazone decreases LPS-induced soluble VCAM-1 in PPARα+/+ but not PPARα−/− mice in vivo.
PPARα+/+ and PPARα−/− mice were treated with pioglitazone or vehicle alone before LPS injection (n = 9/genotype as in Methods). sVCAM-1 levels in PPARα+/+ and PPARα−/− mice are shown at baseline (*p<0.007 PPARα−/− vs. PPARα+/+ mice) and after LPS injection in mice treated with either vehicle or pioglitazone (PPARα+/+, n=9, #p<0.002 LPS/vehicle vs. vehicle; ‡p<0.01 pioglitazone/LPS vs. vehicle/LPS) and in PPARα−/− mice (n=9, †p<0.05 vehicle/LPS vs. vehicle; (NS) non-significant pioglitazone/LPS vs. vehicle/LPS, significance determined using Mann-Whitney test). The mean serum sVCAM-1 concentration of each group ± SD is shown.
DISCUSSION
We present pre-clinical data here that the purportedly PPARγ-specific agonist pioglitazone represses inflammatory responses involving endothelial VCAM-1 and hepatic IκBα in a PPARα-dependent manner both in vitro and in vivo in mice. Indeed, reconstituting PPARα expression in PPARα-deficient endothelial cells restored pioglitazone-mediated inhibition of TNFα-induced VCAM-1 expression. To the best of our knowledge, this is the first demonstration that defined pioglitazone responses in vivo require the presence of PPARα. These studies on the role of PPARα in pioglitazone responses were stimulated by clinical observations that subjects receiving pioglitazone did not demonstrate the progressive increase in sVCAM-1 levels seen in a small cohort of patients with recently diagnosed T2DM receiving placebo alone. Repression of cytokine-induced VCAM-1 expression and triglyceride-lowering are well-established PPARα responses in humans (36, 37). The pre-clinical evidence presented here that pioglitazone at concentrations overlapping those reported in vivo can activate the PPARα-LBD and induce expression of PPARα target genes suggests pioglitazone may directly or indirectly influence PPARα responses, as previously suggested. Interestingly, PPARα-LBD responses to pioglitazone varied considerably among cell types and species, with the greatest PPARα activation evident in EC. This variability may have contributed to pioglitazone’s characterization as being PPARγ-specific (29). Here we have extended prior observations by demonstrating that specific pioglitazone effects in vivo are absent in the PPARα-deficient mouse. Together these findings have potential implications for TZD mechanisms of action, interpreting TZD studies, especially in preclinical models as well as the development of novel PPAR therapeutic agents.
Agonists for the same PPAR isoform can differ significantly in their biologic and clinical effects. In transcriptional profiling and proteomic assays, different PPARγ agonists have both shared and distinct gene expression patterns (38–40). Clinically, both pioglitazone and rosiglitazone lack the irreversible liver failure seen with troglitazone, the first clinically-approved PPARγ agonist (41). In a head-to-head clinical trial, pioglitazone decreased TG significantly while rosiglitazone did not (11), as also suggested by meta-analysis data (42). Although the contribution of differential TZD effects to cardiovascular events remains unclear, our data identifies an additional biologic mechanism that may be involved in pioglitazone responses. Considerable evidence establishes TZDs as limiting inflammation and atherosclerosis in mouse models (44). In clinical studies, both pioglitazone and rosiglitazone lower C-reactive protein (37,45–48), anatomic indicators like carotid intimal medial thickness (48–52) and vessel reactivity (53). In the prospective PROactive study, pioglitazone did not decrease a large, combined primary cardiovascular endpoint although a secondary clinical endpoint of stroke, MI and CV death was improved (54). Various logistical factors have been speculated as contributors to the study’s negative primary endpoint (4). Recently, in the PERISCOPE trial, progression of atherosclerosis was seen on coronary intravascular ultrasound in otherwise well-treated patients randomized to glimiperide but not in those receiving pioglitazone (91). Piogitazine significantly decreased triglycerides and raised HDL in both PROactive and PERISCOPE. No large prospective clinical cardiovascular rosiglitazone trial data is available. Recent meta-analyses have raised concern over a possible increase in cardiovascular risk with rosiglitazone (12–14,55), although with limitations in this data as raised by the authors and others (15, 55). In similar meta-analyses, no increased risk with pioglitazone was found (13). Given changes induced by TZDs on surrogate markers for cardiovascular disease in basic and clinical studies, it remains possible that offsetting adverse cardiovascular effects could exist with these agents. PPARα activation can increase homocysteine levels and serum creatinine levels (56–59). While the VA-HIT study did show decreased cardiovascular events with the putative PPARα agonist gemfibrozil, in FIELD, fenofibrate, a more potent PPARα agonist, did not show a difference in the primary cardiovascular endpoint (60,61). Although the role of pioglitazone-mediated PPARα activation in determining clinical responses remains unclear, our data suggests at the very least that PPARα activation should be considered in interpreting basic science data with this agent.
Both VCAM-1 and IκBα regulate inflammatory responses in atherosclerosis. Endothelial VCAM-1 expression is an important early atherogenic step (2). Circulating levels of VCAM-1 may predict subsequent clinical cardiovascular events (62) while VCAM-1 may be elevated in T2DM, perhaps as a result of the hypertriglyceridemia and/or low HDL-C (34,63,64). Reducing TG levels with fibrates or fish oil, both of which can be considered PPARα activators (67,68), reportedly decrease soluble adhesion molecule levels (32,65,66). VCAM-1 expression is controlled by multiple pathways, including NF-κB and PPARα (69). In vitro, VCAM-1 repression has been reported by some PPARγ agonists but not others, and at drug concentrations that may have PPARγ-independent effects (30–32). Although we found PPARα agonists repressed VCAM-1 in wildtype but not PPARα-deficient EC, rosiglitazone and 15d-PGJ2 had no VCAM-1 effects (20). Differences among reports of PPARγ agonist VCAM-1 effects may involve differences among agents or the cell types under study.
NF-κB activation, which is inhibited by IκBα, increases VCAM-1 expression. PPARα activation induces IκBα (34). Here we found that pioglitazone increased IκBα expression in a PPARα-dependent manner in HSVECs in vitro and in liver in vivo. 15’ deoxy prostaglandin J2 and troglitazone increase IκBα expression but independent of PPARγ (70–72). In contrast, the more selective PPARγ ligand rosiglitazone did not change IκBα expression in human monocyte/macrophages (72,73). In contrast, pioglitazone reportedly increased IκBα levels in peripheral mononuclear cells in human subjects (74). These results are potentially consistent with pioglitazone exerting effects through PPARα as also suggested by studies in which pioglitazone treatment increased expression of PPARα target genes in subcutaneous fat (43). The possibility that these responses derived from pioglitazone activation of PPARα was not discussed (43).
PPAR biology suggests several mechanisms for how agonists for the same PPAR isotype might exert distinct effects. PPARs have a particularly large LBD, even as compared to other nuclear receptors (75). PPAR activation induces a conformational change in the AF2 domain, which allows co-activator recruitment, co-repressor release and formation of the heterodimeric PPAR-RXR complex. These critical determinants of transcriptional responses can vary as a function of different interactions between structurally-distinct PPARγ agonists and the large PPAR LBD (75). Interestingly, other in vitro pharmacologic studies also suggest pioglitazone may activate PPARα (35). Recently, ligand-independent mechanisms influencing PPAR-mediated anti-inflammatory effects have been reported, for example through SUMOylation (76,77). Differences among PPAR-interacting molecules underlie the concept of selective nuclear receptor modulators, as exist for the estrogen receptor and novel PPAR agents in development (78,79). The potential variability among specific PPAR interacting molecules is apparent in the reports of full agonists, selective partial agonists, inverse agonists, antagonists as well as pan-PPAR and dual PPAR agonists (79–82).
Dual PPARα/γ agonists offered the putative clinical benefits of combining HDL-raising/TG-lowering via PPARα with improved insulin sensitivity through PPARγ (79). No dual PPARα/γ agonists have been approved for use (83). Muraglitazar and tesaglitazar reached late stage testing before being abandoned due to adverse effects, including increased cardiovascular events (84,85), raising concerns over dual PPARα/γ agonists as a drug class. Given the evidence that pioglitazone can be used safely (13), our data that pioglitazone may act at least in part through PPARα suggests that selective modulators targeting both PPARα and PPARγ can be safely developed. Moreover, our findings suggest pioglitazone’s description as a PPARγ-specific agonist may need revisiting. Such studies would need to include analysis of pioglitazone’s multiple metabolites, especially ones with biologic activity but unknown PPAR selectivity (86,87). Pioglitazone metabolite production may differ depending on cell types, tissues, species, or genetic variants. Although pioglitazone demonstrates more potent LBD activation of PPARγ than PPARα, the PPARα-LBD activation seen is within a range that could influence biologic responses, especially in EC. LBD activation may also underestimate functional PPAR effects in vivo as a result of mechanisms such as preferential generation, stabilization, or transport of a specific drug metabolite.
Pioglitazone could also regulate PPARα target genes indirectly, for example altering PPARα regulatory proteins or inducing the formation of endogenous PPARα agonists. For example, lipoprotein lipase (LPL), a positively-regulated PPARγ target gene, can generate PPARα ligands through VLDL hydrolysis (21). Increased LPL expression and activity would be associated with lower TG and higher HDL, as occurs with gain of function LPL polymorphisms and after treatment with synthetic PPARα agonists (67,88). Prior work identifies increased LPL-mediated lipolysis as a contributor to pioglitazone’s triglyceride-lowering effects (89). In clinical studies, pioglitazone induces LPL expression and also decreases the natural LPL inhibitor apoCIII (43,89), further supporting possible indirect PPARα activation through increased VLDL hydrolysis. Since both LPL and apoCIII are PPARα-regulated target genes, positive feed-forward mechanisms may amplify these effects (21,90).
Independent of a direct or indirect mechanism, the requirement for PPARα in order for VCAM-1 repression and IκBα induction in vitro and in vivo in mice expands potential mechanisms of action for this agent, at least in vitro and in mouse models. This data also underscores the need to fully understand the effects of both existing and emerging PPAR agonists and their biologically active metabolites. Indeed, the complexity of PPAR biology, the number of variables dictating transcriptional and hence clinical responses, and the fact that agonist structure can determine biologic response argues that the notion of general PPAR-activating drug classes may be limited.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The investigators have received prior funding from GlaxoSmithKline and Takeda Pharmaceuticals, although not for specifically for the support of the pre-clinical murine studies included here. The clinical trial presented here was supported by Takeda Pharmaceuticals (E. Horton, PI).
References
- 1.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001:365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 3.Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest. 2006:1813–1822. doi: 10.1172/JCI29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007:518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- 5.Lindberg M, Astrup A. The role of glitazones in management of type 2 diabetes. A DREAM or a nightmare? Obes Rev. 2007:381–384. doi: 10.1111/j.1467-789X.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 6.Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev. 1995:571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 7.Willson TM, Cobb JE, Cowan DJ, et al. The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem. 1996:665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 8.Physicians Desk Reference. Thomson PDR; 2007. [Google Scholar]
- 9.Blaschke F, Caglayan E, Hsueh WA. Peroxisome proliferator-activated receptor gamma agonists: their role as vasoprotective agents in diabetes. Endocrinol Metab Clin North Am. 2006:561–574. doi: 10.1016/j.ecl.2006.06.001. ix. [DOI] [PubMed] [Google Scholar]
- 10.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg RB, Kendall DM, Deeg MA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005:1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 12.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 13.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and Risk of Cardiovascular Events in Patients With Type 2 Diabetes Mellitus: A Meta-analysis of Randomized Trials. JAMA. 2007:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007:1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 15.Diamond GA, Bax L, Kaul S. Uncertain Effects of Rosiglitazone on the Risk for Myocardial Infarction and Cardiovascular Death. Ann Intern Med. 2007 doi: 10.7326/0003-4819-147-8-200710160-00182. [DOI] [PubMed] [Google Scholar]
- 16.Krall RL. Cardiovascular safety of rosiglitazone. Lancet. 2007:1995–1996. doi: 10.1016/S0140-6736(07)60824-1. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed I, Furlong K, Flood J, Treat VP, Goldstein BJ. Dual PPAR alpha/gamma agonists: promises and pitfalls in type 2 diabetes. Am J Ther. 2007:49–62. doi: 10.1097/01.mjt.0000212890.82339.8d. [DOI] [PubMed] [Google Scholar]
- 18.Rubenstrunk A, Hanf R, Hum DW, Fruchart JC, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta. 2007:1065–1081. doi: 10.1016/j.bbalip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Galkina E, Ley K. Vascular Adhesion Molecules in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2007 doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 20.Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziouzenkova O, Perrey S, Marx N, Bacqueville D, Plutzky J. Peroxisome proliferator-activated receptors. Curr Atheroscler Rep. 2002:59–64. doi: 10.1007/s11883-002-0063-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee SS, Gonzalez FJ. Targeted disruption of the peroxisome proliferator-activated receptor alpha gene, PPAR alpha. Ann N Y Acad Sci. 1996:524–529. doi: 10.1111/j.1749-6632.1996.tb18642.x. [DOI] [PubMed] [Google Scholar]
- 23.Hamdy O, Ledbury S, Mullooly C, et al. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. 2003:2119–2125. doi: 10.2337/diacare.26.7.2119. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Standards of medical care in diabetes--2006. Diabetes Care. 2006 doi: 10.2337/diacare.29.02.06.dc05-1593. S4-42. [DOI] [PubMed] [Google Scholar]
- 26.Ziouzenkova O, Perrey S, Asatryan L, et al. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc Natl Acad Sci U S A. 2003:2730–2735. doi: 10.1073/pnas.0538015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed W, Orasanu G, Nehra V, et al. High-density lipoprotein hydrolysis by endothelial lipase activates PPARalpha: a candidate mechanism for high-density lipoprotein-mediated repression of leukocyte adhesion. Circ Res. 2006:490–498. doi: 10.1161/01.RES.0000205846.46812.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Report of the expert committee on the diagnosis and classification of diabetes mellitus. 2003. [Google Scholar]
- 29.Eckland D, Danhof M. Clinical pharmacokinetics of pioglitazone. Exp Clin Endocrinol Diabetes. 2000;108:S234–S242. [Google Scholar]
- 30.Jackson SM, Parhami F, Xi XP, et al. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999:2094–2104. doi: 10.1161/01.atv.19.9.2094. [DOI] [PubMed] [Google Scholar]
- 31.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rival Y, Beneteau N, Taillandier T, et al. PPARalpha and PPARdelta activators inhibit cytokine-induced nuclear translocation of NF-kappaB and expression of VCAM-1 in EAhy926 endothelial cells. Eur J Pharmacol. 2002:143–151. doi: 10.1016/s0014-2999(01)01589-8. [DOI] [PubMed] [Google Scholar]
- 33.Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 34.Delerive P, Gervois P, Fruchart JC, Staels B. Induction of IkappaBalpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J Biol Chem. 2000:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 35.Sakamoto J, Kimura H, Moriyama S, et al. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun. 2000;278:704–711. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- 36.Ryan KE, McCance DR, Powell L, McMahon R, Trimble ER. Fenofibrate and pioglitazone improve endothelial function and reduce arterial stiffness in obese glucose tolerant men. Atherosclerosis. 2007;194:e123–e130. doi: 10.1016/j.atherosclerosis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Takase H, Nakazawa A, Yamashita S, et al. Pioglitazone produces rapid and persistent reduction of vascular inflammation in patients with hypertension and type 2 diabetes mellitus who are receiving angiotensin II receptor blockers. Metabolism. 2007:559–564. doi: 10.1016/j.metabol.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Gao J, Ann Garulacan L, Storm SM, et al. Identification of in vitro protein biomarkers of idiosyncratic liver toxicity. Toxicol In Vitro. 2004:533–541. doi: 10.1016/j.tiv.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Bottoni P, Giardina B, Martorana GE, et al. A two-dimensional electrophoresis preliminary approach to human hepatocarcinoma differentiation induced by PPAR-agonists. J Cell Mol Med. 2005:462–467. doi: 10.1111/j.1582-4934.2005.tb00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo L, Zhang L, Sun Y, et al. Differences in hepatotoxicity and gene expression profiles by anti-diabetic PPAR gamma agonists on rat primary hepatocytes and human HepG2 cells. Mol Divers. 2006:349–360. doi: 10.1007/s11030-006-9038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson MD, Campbell LK, Campbell RK. Troglitazone: review and assessment of its role in the treatment of patients with impaired glucose tolerance and diabetes mellitus. Ann Pharmacother. 1998:337–348. doi: 10.1345/aph.17046. [DOI] [PubMed] [Google Scholar]
- 42.Chiquette E, Ramirez G, Defronzo R. A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch Intern Med. 2004:2097–2104. doi: 10.1001/archinte.164.19.2097. [DOI] [PubMed] [Google Scholar]
- 43.Bogacka I, Xie H, Bray GA, Smith SR. The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo. Diabetes Care. 2004:1660–1667. doi: 10.2337/diacare.27.7.1660. [DOI] [PubMed] [Google Scholar]
- 44.Plutzky J. Peroxisome proliferator-activated receptors in endothelial cell biology. Curr Opin Lipidol. 2001:511–518. doi: 10.1097/00041433-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002:679–684. doi: 10.1161/01.cir.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 46.Sidhu JS, Cowan D, Kaski JC. The effects of rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, on markers of endothelial cell activation, C-reactive protein, and fibrinogen levels in non-diabetic coronary artery disease patients. J Am Coll Cardiol. 2003:1757–1763. doi: 10.1016/j.jacc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Plutzky J. Peroxisome proliferator-activated receptors as therapeutic targets in inflammation. J Am Coll Cardiol. 2003:1764–1766. doi: 10.1016/j.jacc.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimoto T, Naruse M, Shizume H, et al. Vasculo-protective effects of insulin sensitizing agent pioglitazone in neointimal thickening and hypertensive vascular hypertrophy. Atherosclerosis. 1999:333–340. doi: 10.1016/s0021-9150(99)00085-4. [DOI] [PubMed] [Google Scholar]
- 49.Koshiyama H, Shimono D, Kuwamura N, Minamikawa J, Nakamura Y. Rapid communication: inhibitory effect of pioglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab. 2001:3452–3456. doi: 10.1210/jcem.86.7.7810. [DOI] [PubMed] [Google Scholar]
- 50.Sidhu JS, Kaposzta Z, Markus HS, Kaski JC. Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients without diabetes mellitus. Arterioscler Thromb Vasc Biol. 2004:930–934. doi: 10.1161/01.ATV.0000124890.40436.77. [DOI] [PubMed] [Google Scholar]
- 51.Mazzone T, Meyer PM, Feinstein SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. Jama. 2006:2572–2581. doi: 10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- 52.Hedblad B, Zambanini A, Nilsson P, Janzon L, Berglund G. Rosiglitazone and carotid IMT progression rate in a mixed cohort of patients with type 2 diabetes and the insulin resistance syndrome: main results from the Rosiglitazone Atherosclerosis Study. J Intern Med. 2007:293–305. doi: 10.1111/j.1365-2796.2007.01767.x. [DOI] [PubMed] [Google Scholar]
- 53.Dandona P, Aljada A, Chaudhuri A. Vascular reactivity and thiazolidinediones. Am J Med. 2003:81S–86S. doi: 10.1016/j.amjmed.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 54.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 55.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 56.Dierkes J, Westphal S, Luley C. Serum homocysteine increases after therapy with fenofibrate or bezafibrate. Lancet. 1999:219–220. doi: 10.1016/S0140-6736(99)02153-4. [DOI] [PubMed] [Google Scholar]
- 57.Lipscombe J, Lewis GF, Cattran D, Bargman JM. Deterioration in renal function associated with fibrate therapy. Clin Nephrol. 2001:39–44. [PubMed] [Google Scholar]
- 58.Luc G, Jacob N, Bouly M, Fruchart JC, Staels B, Giral P. Fenofibrate increases homocystinemia through a PPARalpha-mediated mechanism. J Cardiovasc Pharmacol. 2004:452–453. doi: 10.1097/00005344-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 59.Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol. 2007:3C–18C. doi: 10.1016/j.amjcard.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 61.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 62.Mulvihill NT, Foley JB, Crean P, Walsh M. Prediction of cardiovascular risk using soluble cell adhesion molecules. Eur Heart J. 2002:1569–1574. doi: 10.1053/euhj.2002.3188. [DOI] [PubMed] [Google Scholar]
- 63.Abe Y, El-Masri B, Kimball KT, et al. Soluble cell adhesion molecules in hypertriglyceridemia and potential significance on monocyte adhesion. Arterioscler Thromb Vasc Biol. 1998:723–731. doi: 10.1161/01.atv.18.5.723. [DOI] [PubMed] [Google Scholar]
- 64.Calabresi L, Gomaraschi M, Villa B, Omoboni L, Dmitrieff C, Franceschini G. Elevated soluble cellular adhesion molecules in subjects with low HDL-cholesterol. Arterioscler Thromb Vasc Biol. 2002:656–661. doi: 10.1161/hq0402.105901. [DOI] [PubMed] [Google Scholar]
- 65.Xu X, Otsuki M, Saito H, et al. PPARalpha and GR differentially down-regulate the expression of nuclear factor-kappaB-responsive genes in vascular endothelial cells. Endocrinology. 2001:3332–3339. doi: 10.1210/endo.142.8.8340. [DOI] [PubMed] [Google Scholar]
- 66.Okapcova J, Gabor D. The levels of soluble adhesion molecules in diabetic and nondiabetic patients with combined hyperlipoproteinemia and the effect of ciprofibrate therapy. Angiology. 2004:629–639. doi: 10.1177/00033197040550i604. [DOI] [PubMed] [Google Scholar]
- 67.Pineda Torra I, Gervois P, Staels B. Peroxisome proliferator-activated receptor alpha in metabolic disease, inflammation, atherosclerosis and aging. Curr Opin Lipidol. 1999:151–159. doi: 10.1097/00041433-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 68.Sethi S, Ziouzenkova O, Ni H, Wagner DD, Plutzky J, Mayadas TN. Oxidized omega-3 fatty acids in fish oil inhibit leukocyte-endothelial interactions through activation of PPAR alpha. Blood. 2002:1340–1346. doi: 10.1182/blood-2002-01-0316. [DOI] [PubMed] [Google Scholar]
- 69.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 70.Rossi A, Kapahi P, Natoli G, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 71.Ward C, Dransfield I, Murray J, Farrow SN, Haslett C, Rossi AG. Prostaglandin D2 and its metabolites induce caspase-dependent granulocyte apoptosis that is mediated via inhibition of I kappa B alpha degradation using a peroxisome proliferator-activated receptor-gamma-independent mechanism. J Immunol. 2002:6232–6243. doi: 10.4049/jimmunol.168.12.6232. [DOI] [PubMed] [Google Scholar]
- 72.Guyton K, Bond R, Reilly C, Gilkeson G, Halushka P, Cook J. Differential effects of 15-deoxy-delta(12,14)-prostaglandin J2 and a peroxisome proliferator-activated receptor gamma agonist on macrophage activation. J Leukoc Biol. 2001:631–638. [PubMed] [Google Scholar]
- 73.Mohanty P, Aljada A, Ghanim H, et al. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004:2728–2735. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- 74.Klotz L, Schmidt M, Giese T, et al. Proinflammatory stimulation and pioglitazone treatment regulate peroxisome proliferator-activated receptor gamma levels in peripheral blood mononuclear cells from healthy controls and multiple sclerosis patients. J Immunol. 2005:4948–4955. doi: 10.4049/jimmunol.175.8.4948. [DOI] [PubMed] [Google Scholar]
- 75.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 76.Pascual G, Fong AL, Ogawa S, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghisletti S, Huang W, Ogawa S, et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dutertre M, Smith CL. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther. 2000:431–437. [PubMed] [Google Scholar]
- 79.Fievet C, Fruchart JC, Staels B. PPARalpha and PPARgamma dual agonists for the treatment of type 2 diabetes and the metabolic syndrome. Curr Opin Pharmacol. 2006:606–614. doi: 10.1016/j.coph.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 80.Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- 81.Seimandi M, Lemaire G, Pillon A, et al. Differential responses of PPARalpha, PPARdelta, and PPARgamma reporter cell lines to selective PPAR synthetic ligands. Anal Biochem. 2005:8–15. doi: 10.1016/j.ab.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Hamuro Y, Coales SJ, Morrow JA, et al. Hydrogen/deuterium-exchange (H/D-Ex) of PPARgamma LBD in the presence of various modulators. Protein Sci. 2006:1883–1892. doi: 10.1110/ps.062103006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henke BR. Peroxisome proliferator-activated receptor alpha/gamma dual agonists for the treatment of type 2 diabetes. J Med Chem. 2004:4118–4127. doi: 10.1021/jm030631e. [DOI] [PubMed] [Google Scholar]
- 84.Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. Jama. 2005:2581–2586. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]
- 85.Hellmold H, Zhang H, Andersson U, et al. Tesaglitazar, a PPARalpha/gamma agonist, induces interstitial mesenchymal cell DNA synthesis and fibrosarcomas in subcutaneous tissues in rats. Toxicol Sci. 2007:63–74. doi: 10.1093/toxsci/kfm094. [DOI] [PubMed] [Google Scholar]
- 86.Lin ZJ, Ji W, Desai-Krieger D, Shum L. Simultaneous determination of pioglitazone and its two active metabolites in human plasma by LCMS/MS. J Pharm Biomed Anal. 2003:101–108. doi: 10.1016/s0731-7085(03)00344-3. [DOI] [PubMed] [Google Scholar]
- 87.Shen Z, Reed JR, Creighton M, et al. Identification of novel metabolites of pioglitazone in rat and dog. Xenobiotica. 2003:499–509. doi: 10.1080/0049825031000085951. [DOI] [PubMed] [Google Scholar]
- 88.Wang XL, McCredie RM, Wilcken DE. Common DNA polymorphisms at the lipoprotein lipase gene. Association with severity of coronary artery disease and diabetes. Circulation. 1996:1339–1345. doi: 10.1161/01.cir.93.7.1339. [DOI] [PubMed] [Google Scholar]
- 89.Nagashima K, Lopez C, Donovan D, et al. Effects of the PPARgamma agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. J Clin Invest. 2005:1323–1332. doi: 10.1172/JCI23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Staels B, Vu-Dac N, Kosykh VA, et al. Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J Clin Invest. 1995:705–712. doi: 10.1172/JCI117717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.