Abstract
Chemical communication and perception strategies between plants are highly sophisticated but are only partly understood. Among the different interactions, the suppressive interaction of a class of chemicals released by one plant through root exudates against the neighbouring plants (allelopathy) have been implicated in the invasiveness of many exotic weedy species. Phragmites australis (common reed) is one of the dominant colonizers of the North American wetland marshes and exhibits invasive behavior by virtually replacing the entire native vegetation in its niche. Recently, by adopting a systematic bioassay driven approach we elucidated the role of root derived allelopathy as one of the important mechanisms by which P. australis exerts its invasive behavior. Additionally, our recent preliminary data indicates the involvement of rhizobacterial signaling in the invasive success of P. australis. A better understanding of biochemical weaponry used by P. australis will aid scientists and technologists in addressing the impact of root secretions in invasiveness of weedy species and thus promote a more informed environmental stewardship.
Key words: Phragmites australis, roots, phytotoxicity, reactive oxygen species, microtubules, microcosm
Introduction
Phragmites australis (Cav.) Trin. ex Steudel, is a perennial graminaceous plant that propagates mainly through rhizomes and, at low frequency, by seeds.1 P. australis is widely distributed, ranging all over Europe, Asia, Africa, America and Australia, however, the origin of the species is unclear though some reports points towards Eurasian region. According to archaeological records, it has been present in the United States for at least 40,000 years and is considered a native species.2 In the United States P. australis is found to occur in every state except Arkansas (probably present but undocumented) and is predominantly seen along the borders of lakes, ponds and rivers.3 The distribution and relative abundance of P. australis has increased dramatically over the last 150 years.4 Presently, P. australis represents one of the most abundant plant species in the US coastal wetlands and is considered as an indicator of wetland disturbance.5 The invasion and establishment of monocultures of P. australis, has resulted in the changed ecosystem processes and associated detrimental impacts on native biodiversity and wild life. Some of the reported negative impacts of P. australis include the effect on the larval and juvenile fish population.6 Effects on the abundance in numbers of ecological specialists and rare species such as willet, seaside sparrow, and sharp-tailed sparrow.7 Plant community studies in the P. australis dominated ecosystems have shown that Phragmites are not conducive to the establishment of other plant species and that colonization of disturbed wetland areas by this plant usually ensures the development of dense stands.8–11 The present review is mainly focused on the discussion of the various mechanisms by which P. australis exerts its invasive behavior to successfully colonize and replace the native biodiversity.
Eco-Geographical Factors Influencing P. australis Invasion
Introduction of an invasive species to a new habitat creates more competition for space and resources with native species. Traditionally, the introduced species are found to be more aggressive because of the nonavailability of predators in the new habitat.12 P. australis is now considered as one of the major invasive species causing a considerable negative impact on native biodiversity.13 One of the earliest reasons attributed for the P. australis invasion includes its ability to alter soil properties, reduce salinity, low water level at the surface, pronounced micro topographic relief and higher redox potentials.14 Various reasons, such as human activities that lead to ecological disturbances and stresses such as pollution, changes in hydrologic regimes and increased soil salinity have also been proposed as causes for the rapid expansion of P. australis.15 It has been reported by both univariate and aggregate (multidimensional scaling) analyses of plant community composition that Phragmites dominance in developed salt marshes resulted in an almost three-fold decrease in plant species richness.16 However, none of these eco-geographical factors have been experimentally proven conclusively.17 In addition, a number of edaphic factors also have been suggested for the invasive success of P. australis, but were not accepted due to a lack of experimental evidence.18 Furthermore, population explosion of P. australis is often thought of being facilitated by changes in land use patterns, urbanization and eutrophication.17 However, the very same factors are thought to cause declines of P. australis in Europe.19 While, P. australis aggressively colonizes and replaces the native vegetation, native plants on the other hand try to make adjustments in their anatomical/phenotypic properties to compete against P. australis. In an interesting study conducted using a floating peat mat, it was found that under the P. australis canopy, Menyanthes trifoliata adjusted its phenotypic properties to the conditions of decreased light by increasing leaf blade area, decreasing leaf blade thickness and elongating petioles.20 In spite of the scanty observations of few such anatomical and ecological adaptations by the native species, they still fail to out compete P. australis. Despite progress towards understanding the ecology of invasive plants and recipient communities,21–23 little headway has been made in identifying the genetic and biochemical changes (synthesis of phytotoxins) responsible for invasiveness.4,24 Yet this information is required to answer the fundamental question of what makes an invasive weed; i.e., plants that are unusually persistent, pernicious and optimally adapted to agro-ecosystems.25
Genetic Structure of P. australis Population and Contribution to Invasiveness
The most common context in which invasion has been attributed to the evolution of an introduced species is the “evolution of increased competitive ability” or EICA hypothesis, proposed by Blossey and Nötzold.26 They suggested that release from natural enemies would result in plants that evolve to allocate less to herbivore defense27 and reallocate this freed energy and resources to growth. Greater growth would make these new genotypes more competitive than their predecessors in the native range. A number of studies have shown that invasive genotypes are larger or more reproductive than conspecifics in their native range,26,28,29 but conclusive support for full causal processes proposed for EICA is rare.30
The early studies involving the analysis of peat core suggested that P. australis as not a common member of mixed tidal wetland plant communities in North America for at least 3,000 years.31,32 It was during the 19th but particularly in the late 20th century, P. australis has been observed invading fresh and brackish water wetlands with eventual expansion of its range and abundance in North America. Moreover, studies during late eighties and early nineties started suggesting that the invasiveness of P. australis is attributable to introduction of more aggressive European genotypes33–35 but until recently, little information was available to support this hypothesis. The existence of introduced European haplotypes along with the native North American haplotypes was confirmed recently by more detailed genetic studies.4 The leading explanation for the rapid expansion of invasive Phragmites populations in North America is the observation that they are genetically different from native Phragmites.4 Chloroplast DNA (cpDNA) analysis has shown that invasive populations possess a single cpDNA haplotype (M) which is also widespread in Europe and Asia, while thirteen native North American Phragmites haplotypes have now been documented.4 These data are supported further by nuclear microsatellite DNA analysis which show similar patterns in genetic differentiation36 and morphological differences which distinguish native and introduced Phragmites in North America.37 When grown under the same conditions, introduced Phragmites has significantly higher biomass, both above and belowground, than native Phragmites38 and similar patterns have been observed under field conditions.39,40 Importantly, we do not have any concrete evidence that suggests that North American populations of Phragmites have evolved to be larger and better competitors than populations in Europe and thus meeting no predictions of EICA. However, recent work suggests that introduced North American populations of Phragmites appear to possess a potent “novel biochemical weapon” that allows them to gain advantage over native North American species.
Novel Weapons Involved in P. australis Invasion?
More recently, the possibility of disrupted coadaptation as a driver of invasion has been considered in the context of the “novel weapons hypothesis” (NWH).41–43 This is the idea that some invaders may succeed because they possess unique allelopathic, defensive or antimicrobial biochemistry to which naïve natives have not adapted. Like EICA, there are a number of studies that support this hypothesis.22,42,44–47 Apart from the edaphic, environmental and genetic factors, allelopathic interaction among salt marsh plants has been suggested as a possible reason for the zonation in the marsh exhibited by P. australis and other species.48 However, the initial experiments with P. australis rhizosphere washings did not cause any phytotoxic affect on two salt marsh plant species, Distichlis spicata or Scirpus robustus. Three triterpenoids (β-amanacin, taraxerol, taraxeron) and a flavone (tricin) have been identified from the aerial portions of P. australis.49,50 In addition, various phytochemicals were extracted from P. communis (earlier name for P. australis) and tested for their anti-algal effect on different algal species.51 The screen identified the allelopathic effect of ethyl-2-methylacetoacetate against the algal species Chlorella pyrenoidosa and Microcystis aeruginosa.51 However, none of these identified chemicals were tested for their allelochemical activity on other seed plant species. The root-derived allelopathy was not studied in detail in P. australis; this may be partially because of the difficulty involved with isolation and characterization of root secretions. In our study we addressed this issue by establishing an in vitro system, whereby P. australis grown in vitro can secrete allelochemicals into a growth medium.52 Further, the root exudates from invasive haplotype of P. australis P38 showed phytotoxic effect on a number of plant species including the P. australis associated Spartina alterniflora and the model plant Arabidopsis thaliana.52 The phytotoxic effect of the root exudates was mainly by the inhibition of root growth initiated by the death of the root starting from the tip to central elongation zone.52 The allelopathic effect was subsequently established by the in vivo pot experiments where P. australis exotic haplotype completely suppressed the germination and growth of A. thaliana.52 However when activated charcoal (which acts as a sink and adsorbs all the root secreted allelochemicals) was included, no suppression of germination and growth was observed. Further the bioassay-drives fractionation and HPLC, LC-MS analysis of the root exudates revealed that the active ingredient in the root exudates as gallic acid (3,4,5-trihydroxybenzoic acid). Further, the gallic acid identified in the root exudates of P. australis was highly phytotoxic to A. thaliana and other plant species tested. Gallic acid particularly induced rhizotoxicity similar to crude root exudates leading to severe inhibition of root growth and disrupting the total root architecture. Gallic acid, a phenolic compound found in a number of dicot species53,54 (e.g., oak, birch, tea, grape wine and sumac), is a main constituent of gallo-tannins. Though, the gallic acid biosynthetic pathway is still debated, it has been shown that gallic acid is synthesized directly from its precursor 5-dehydroshikimate in the leaves of Rhus typhina.55 Although, the presence of gallic acid has been shown in monocots,51,56 its biosynthetic pathway has not been deduced in these species. It may be because of the presence of an altogether different but specific biosynthetic pathway in monocots. In addition, the presence of other phenolic acids such as elagic acid in P. communis (former name for P. australis) supports this view.51 Since gallic acid is a phenolic compound, and such compounds are known to generate reactive oxygen species (ROS),57 we hypothesized that gallic acid might trigger ROS generation that could lead to the cell death cascade in the treated roots. As per our hypothesis, when we checked for ROS generation, the gallic acid treated Arabidopsis Col-0 seedlings showed a significantly higher basal ROS level on the root surface compared to untreated controls. Further the generated ROS was able to be quenched by the inclusion of the well known ROS quencher the ascorbic acid. Therefore, the phytotoxicity of gallic acid exhibited by the treated roots appears to be triggered by elevated levels of ROS. These results further prompted us to investigate the cellular role of ROS in causing root death. Since the gallic acid treatment resulted in the complete collapse of the roots, we suspected that its role was in disrupting the microtubule architecture. Accordingly, we imaged the gallic acid-treated roots of A. thaliana-CTD-PAPK1-GFP, a transgenic line carrying a specific GFP tag for visualizing microtubule architecture. Surprisingly, increased microtubule disruption was associated with increased ROS levels over a time period. The significant role of ROS in bringing about microtubule disruption was strongly supported when the plants incubated with the AsA,58 a ROS quencher and antioxidant, showed very little or no microtubule disruption. The gallic acid-treated plants exhibited a considerable amount of recovery when incubated with AsA. ROS has been implicated in the signaling and induction of various plant processes starting from pathogenesis59 to various other stimuli such as gravity response, abscissic acid, auxin, gibberellic acid, UV-B light and nodulation (nod) factors.60–62 An important question here is how do ROS modify the down stream targets leading to phytotoxicity? The response reported in recent study52 might be occurring through ROS-mediated modification of the down stream signaling proteins leading to altered gene expression and cell death. The ROS generated by phenols have been implicated in the microtubule destruction and associated cell death in human cancer cell lines.63 A polyphenol, phenol, [(4-dihydroxyphenyl)-3-hydroxy-(4′-hydroxyphenyl) 1-propanone (β-hydroxy-DHP)], isolated from licorice root (Glycyrrhiza glabra) has been reported to inhibit microtubule assembly in human breast and prostate cancer cell lines.64 However, to the best of our knowledge, there are no reports on a plant rhizo-secreted toxin damaging the microtubule net work of the root cells of other plants. Overall, series of experimental results reported in our study indicate that P. australis secretes a phenolic compound gallic acid into the rhizosphere. The rhizo-secreted gallic acid bears an allelopathic effect on the tested plants both in vitro and in pot experiments under green house conditions. Further, the gallic acid inhibited plant growth through ROS-mediated cell death associated with destruction of the root microtubule assembly presents a strong case for the existence of root derived allelopathy in P. australis.
Are there Other Partners in Crime?
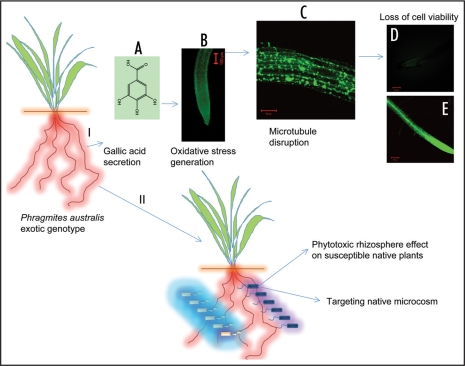
In addition to ecogeographical, genetic and secretion of phytotoxins in to the rhizosphere, one can think of other partners such as a rhizosphere and endophytic microorganisms, which might aid in its endeavor of conquering vast geographical area. The grass species and endophytic fungal and bacterial symbiosis has been studied in considerable detail in a number of species.65–68 However, the involvement of bacterial endophytes and epiphytic root colonizers which are specifically associated with roots of exotic P. australis needs to be unraveled. Further, the testing of this hypothesis should answer the questions such as whether rhizospheric microbes involved in triggering the invasive behavior of P. australis. If so are there a differential colonization patterns between the invasive and exotic lines? Finally does the root associated rhizospheric bacteria synthesize an additional phytotoxin to elevate the allelopathic effect of P. australis. The role of biochemical and rhizospheric microcosm signaling in between P. australis and the native plants has been depicted in Figure 1.
Figure 1.
(I) Root secreted allelochemical, gallic acid (A) triggers a self destructive pathway in the susceptible plant roots by elevating the basal reactive oxygen species (ROS) levels (B) which in turn disrupts the microtubule network (C) leading to complete rhizotoxicity and death [loss of fluorescence (D)] of the treated roots (D). (E) shows a control root exhibiting viability. (II) The P. australis exotic haplotypes root associated microorganism response in elevating the rhizotoxicity on the susceptible plant roots.
Conclusions
The studies so far on the invasive mechanisms of P. australis emphasized on various reasons starting from ecological, edaphic, anthropogenic and genetic. Our recent study established that P. australis indeed releases a rhizotoxin in to the rhizosphere thorough root exudates which kills the plants in its vicinity. We also show that the secreted toxin initiates a suicidal cell death cascade in the susceptible plants by elevating the ROS levels and eventual destruction of microtubules. Our studies, which are still preliminary, also indicate the contribution of root associated bacteria for P. australis invasion. As to the consequences of root secreted toxin activity, we will expand upon our knowledge of direct effects on plant roots to analyze the effects of phytotoxin secretions on entire native microcosms. Root-derived biochemical changes coupled with microbial diversity cataloguing reported in only a few groups of invasive plants will provide vital clues regarding how species become invasive and may lead to environmental and economic benefits through better management of invasive species.
Abbreviations
- EICA
evolution of increased competitive ability
- HPLC
high performance liquid chromatography
- LC-MS
liquid chromatograph-mass spectrometry
- cpDNA
chloroplast deoxyribonucleic acid
- NWH
novel weapons hypothesis
- ROS
reactive oxygen species
- GFP
green fluorescent protein
- AsA
ascorbic acid
- UV-B
ultraviolet B
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5279
References
- 1.Coops H, Velde G. Seed dispersal, germination and seedling growth of six helophyte species in relation to water-level zonation. Freshwater Biol. 1995;34:13–20. [Google Scholar]
- 2.Hansen RM. Shasta ground sloth food habits, Rampart Cave, Arizona. Paleobiology. 1978;4:302–319. [Google Scholar]
- 3.Harrington HD. Manual of the plants of Colorado. Denver, CO.: Sage Books; 1964. p. 666. [Google Scholar]
- 4.Saltonstall K. Cryptic invasion by a nonnative genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci USA. 2002;99:2445–2449. doi: 10.1073/pnas.032477999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blossey B. Native to North America or introduced or both? Ithaca, NY: Ag Web, Cornell University; 2002. p. 3. ( http://www.invasiveplants.net/P. australis/phrag/natint.htm) [Google Scholar]
- 6.Able KW, Hagan SM. Effects of common reed (Phragmites australis) invasion on marsh surface macro-fauna: Response of fishes and decapod crustaceans. Estuaries. 2000;23:633–646. [Google Scholar]
- 7.Benoit LK, Askins RA. Impact of the spread of Phragmites on the distribution of birds in tidal Connecticut marshes. Wetlands. 1999;19:194–208. [Google Scholar]
- 8.Chambers RM, Meyerson LA, Saltonstall K. Expansion of Phragmites australis into tidal wetlands of North America. Aquat Bot. 1999;64:261–273. [Google Scholar]
- 9.Meyerson LA, Saltonstall K, Windham L, Kiviat E, Findlay SA. Comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetlands Ecol Management. 2000;8:89–103. [Google Scholar]
- 10.Warren RS, Fell PE, Grimsby JL, Buck EL, Rilling GC, Fertik RA. Rate, patterns, and impacts of Phragmites australis expansion and effects of experimental Phragmites control on vegetation, macroinvertebrates, and fish within tidelands of the lower Connecticut River. Estuaries. 2001;24:90–107. [Google Scholar]
- 11.Ailstock MS, Norman CM, Bushmann PJ. Common reed, Phragmites australis: Controland effects upon biodiversity in freshwater nontidal wetlands. Restoration Ecology. 2001;9:49–59. [Google Scholar]
- 12.Inderjit, Callaway RM, Vivanco JM. Can plant biochemistry contribute to understanding of invasion ecology? Trends Plant Sci. 2006;11:574–580. doi: 10.1016/j.tplants.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Marris E. Shoot to kill. Nature. 2005;438:272–273. doi: 10.1038/438272a. [DOI] [PubMed] [Google Scholar]
- 14.Windham L, Lathrop R. Effects of Phragmites australis (common reed) invasion on above-ground biomass and soil properties in brackish tidal marsh of Mullica River, New Jersey. Estuaries. 1999;22:927–935. [Google Scholar]
- 15.Minchinton TE. Disturbance by wrack facilitates spread of P. australis in a coastal marsh. J Exp Marine Biol Ecol. 2002;281:89–107. [Google Scholar]
- 16.Silliman BR, Bertness MD. Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes. Conservation Biology. 2004;18:1424–1434. [Google Scholar]
- 17.Marks M, Lapin B, Randall J. Phragmites australis (P. communis): Threats, management, and monitoring. Natural Areas Journal. 1994;14:285–294. [Google Scholar]
- 18.Chambers RM, Osgood DT, Kalapasev N. Hydrologic and chemical control of P. australis growth in tidal marshes of SW Connecticut, USA. Marine Ecological Progressive Series. 2002;9:83–91. [Google Scholar]
- 19.van der Putten W. Die-back of Phragmites australis in European wetlands: An overview of the European research program on reed die-back and progression (1993–1994) Aquatic Bot. 1997;59:263–275. [Google Scholar]
- 20.Haraguchi A. Phenotypic and phenological plasticity of an aquatic macrophyte Menyanthes trifoliata L. J Plant Res. 1993;106:31–35. [Google Scholar]
- 21.Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O'Neil P, Parker IM, Thompson JN, Weller SG. The population biology of invasive species. Annu Rev Ecol Systematics. 2001;32:305–332. [Google Scholar]
- 22.Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science. 2003;301:1377–1380. doi: 10.1126/science.1083245. [DOI] [PubMed] [Google Scholar]
- 23.Callaway RM, Ridenour WM. Novel weapons: Invasive success and the evolution of increased competitive ability. Frontiers Ecol Environ. 2004;2:436–443. [Google Scholar]
- 24.Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- 25.Basu C, Halfhill MD, Mueller TC, Stewart CN., Jr Weed genomics: New tools to understand weed biology. TIPS. 2004;9:391–398. doi: 10.1016/j.tplants.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Blossey B, Notzold R. Evolution of increased competitive ability in invasive nonindigenous plants-a hypothesis. J Eco. 1995;83:887–889. [Google Scholar]
- 27.Janzen DH. Studies in Biology. London: Edward Arnold; 1975. Ecology of plants in tropics; p. 8. [Google Scholar]
- 28.Siemann E, Rogers WE. Genetic differences in growth of an invasive tree species. Ecol Lett. 2001;4:514–518. [Google Scholar]
- 29.Jakobs G, Weber E, Edwards PJ. Introduced plants of the invasive Solidago gigantea (Asteraceae) are larger and grow denser than conspecifics in the native range. Divers Distrib. 2004;10:11–19. [Google Scholar]
- 30.Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D. Phenotypic and genetic differentiation in native versus introduced plant populations. Oecologia. 2005;144:1–11. doi: 10.1007/s00442-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 31.Niering WA, Warren RS, Weymouth C. Our dynamic tidal marshes: Vegetation changes as revealed by peat analysis. 1977 (Connecticut Arboretum Bulletin No. 22) [Google Scholar]
- 32.Orson RA, Warren RS, Niering WA. Development of a tidal marsh in a New England river valley. Estuaries. 1987;10:20–27. [Google Scholar]
- 33.Metzler K, Rosza R. Additional notes on the tidal wetlands of the Connecticut River. Newsletter of the Connecticut Botanical Society. 1987;15:1–6. [Google Scholar]
- 34.Tucker GC. The genera of Arundinoidea (Gramineae) in the southeastern United States. J Arnold Arboretum. 1990;71:14–171. [Google Scholar]
- 35.Besitka MAR. An Ecological and Historical Study of Phragmites australis Along the Atlantic Coast. Philadelphia, PA: Master's Thesis. Drexel University; 1996. [Google Scholar]
- 36.Saltonstall K. Microsatellite variation within and among North American lineages of Phragmites australis. Mol Ecol. 2003;12:1689–1702. doi: 10.1046/j.1365-294x.2003.01849.x. [DOI] [PubMed] [Google Scholar]
- 37.Saltonstall K, Peterson PM, Soreng RJ. Recognition of Phragmites australis subsp. americanus (Poaceae: Arundinaceae) in North America: Evidence from morphological and genetic analyses. SIDA. 2004;21:683–692. [Google Scholar]
- 38.Saltonstall K, Stevenson JC. The effect of nutrients on seedling growth of native and introduced Phragmites australis. Aquatic Botany. 2007;86:331–336. [Google Scholar]
- 39.League MT, Colbert EP, Seliskar DM, Gallagher JL. Rhizome growth dynamics of native and exotic haplotypes of Phragmites australis (common reed) Estuaries Coasts. 2006;29:269–276. [Google Scholar]
- 40.Meadows RE, Saltonstall K. Distribution of native and introduced Phragmites australis in freshwater and oligohaline tidal marshes of the Delmarva peninsula and southern New Jersey. J Torrey Bot Soc. 2007 In press. [Google Scholar]
- 41.Rabotnov TA. Importance of the evolutionary approach to the study of allelopathy. Translated from Russian in Ékologia. 1982;3:5–8. [Google Scholar]
- 42.Callaway RM, Aschehoug ET. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science. 2000;290:521. doi: 10.1126/science.290.5491.521. [DOI] [PubMed] [Google Scholar]
- 43.Callaway RM, Ridenour WM. Novel weapons: Invasive success and the evolution of increased competitive ability. Frontiers in Ecol Environment. 2004;2:436–443. [Google Scholar]
- 44.Vivanco JM, Bais HP, Stermitz FR, Callaway RM. Root allelochemistry strongly contributes to Centaurea diffusa invasive behavior. Ecology Lett. 2004;7:285–292. [Google Scholar]
- 45.Cappuccino N, Carpenter D. Invasive exotic plants suffer less herbivory than noninvasive exotic plants. Biology Lett. 2005;1:435–438. doi: 10.1098/rsbl.2005.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cappuccino N, Arnason JT. Novel chemistry of invasive exotic plants. Biology Lett. 2006;2:189–193. doi: 10.1098/rsbl.2005.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC, Hallett SG, Rati D, Klironomos JN. Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLOS Biol. 2006;4/5:e140. doi: 10.1371/journal.pbio.0040140. (DOI: http://dx.doi.org/10.1371/journalpbio.0040140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drifmeyer JE, Zieman JC. Germination enhancement and inhibition of Distichlis spicata and Scirpus robustus seeds from Viriginia. Estuaries. 1979;2:16–21. [Google Scholar]
- 49.Ohmoto T. Triterpenoids and the related compounds from graminaceous plants. Yakugaku Zasshi. 1969;89:1682–1687. [PubMed] [Google Scholar]
- 50.Kaneta MM, Sugiyama N. The constituents of Arthraxon hispidus Makina, Miscanthus tinctorius Hackel, Miscanthus Sinensis Andress, and Phragmites communis Trinius. Japan: Bulletin of Chemical Society. 1972;45:528–531. [Google Scholar]
- 51.Li FM, Hu HY. Isolation and characterization of a novel antialgal allelochemical from Phragmites communis. Appl Environ Microbiol. 2005;11:6545–6553. doi: 10.1128/AEM.71.11.6545-6553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudrappa T, Bonsall J, Gallagar J, Seliskar DM, Bais HP. Root secreted allelochemical in noxious weed Phragmites australis deploys reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J Chem Ecol. 2007;33:1898–1918. doi: 10.1007/s10886-007-9353-7. [DOI] [PubMed] [Google Scholar]
- 53.Barman K, Rai SN. Role of tannins in plant-animal relationship-a review. Indian J Dairy Sci. 2000;53:390–410. [Google Scholar]
- 54.Kraus T, Dahlgren RA, Zasoski RJ. Tannins in nutrient dynamics of forest ecosystems-a review. Plant Soil. 2003;256:41–66. [Google Scholar]
- 55.Werner RA, Rossmann A, Schwarz C, Bacher A, Schmidt HL, Eisenreich W. Biosynthesis of gallic acid in Rhus typhina: Discrimination between alternative pathways from natural oxygen isotope abundance. Phytochem. 2004;65:2809–2813. doi: 10.1016/j.phytochem.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 56.Iqbal Z, Hiradate S, Noda A, Isojima S, Fujii Y. Allelopathic activity of buckwheat: Isolation and characterization of phenolics. Weed Sci. 2003;51:657–662. [Google Scholar]
- 57.Muzandu K, Shaban Z, Ishizuka M, Kazusaka A, Fujita S. Nitric oxide enhances catechol estrogen-induced oxidative stress in LNCaP cells. Free Radical Res. 2005;39:389–398. doi: 10.1080/10715760400029710. [DOI] [PubMed] [Google Scholar]
- 58.Chen Z, Gallie DR. Increasing tolerance to ozone by elevating foliar ascorbic acid confers greater protection against ozone than increasing avoidance. Plant Physiol. 2005;138:1673–1689. doi: 10.1104/pp.105.062000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torres MA, Dangl JL, Jones JDG. Arabidopsis gp91PphoxP homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, Zhang L, Dong F, Gallo J, Galbraith DW, Song CP. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001;126:1438–1448. doi: 10.1104/pp.126.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mackerness AH, John CF, Jordan B, Thomas B. Early signaling components in ultraviolet-B responses: Distinct role for different reactive oxygen species and nitric oxide. FEBS Lett. 2001;489:237–242. doi: 10.1016/s0014-5793(01)02103-2. [DOI] [PubMed] [Google Scholar]
- 62.D'Haeze W, Rycke RD, Mathis R, Goormachtig S, Pagnotta S, Verplancke C, Capoen W, Holsters M. Reactive oxygen species and ethylene play a positive role in lateral root base nodulation of a semi aquatic legume. Proc Natl Acad Sci USA. 2003;100:11789–11794. doi: 10.1073/pnas.1333899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Makoto I, Nahoko S, Kazutop I, Hiroyuki T, Yukio PO. Role of reactive oxygen species in gallic acid-induced apoptosis. Biol Pharma Bulletin. 2000;23:1153–1157. doi: 10.1248/bpb.23.1153. [DOI] [PubMed] [Google Scholar]
- 64.Rafi MM, Vastano BC, Zhu N, Ho CT, Ghai G, Rosen RT, Gallo MA, DiPaola RS. Novel polyphenol molecule isolated from licorice root (Glycyrrhiza glabra) induces apoptosis, G2/M cell cycle arrest, and Bcl-2 phosphorylation in tumor cell lines. J Agric Food Chem. 2002;50:677–684. doi: 10.1021/jf010774e. [DOI] [PubMed] [Google Scholar]
- 65.Barrow J, Lucero M, Reyes-Vera I, Havstad C. Endosymbiotic fungi structurally integrated with leaves reveals a lichenous condition of C4 grasses. In Vitro Cellular and Developmental Biology - Plant. 2007;43:65. [Google Scholar]
- 66.Krauss J, Härri SA, Bush L, Husi R, Bigler L, Power SA, Müller CB. Effects of fertiliser, fungal endophytes and plant cultivar on the performance of insect herbivores and their natural enemies. Functional Ecology. 2007;21:107–116. [Google Scholar]
- 67.Rasmussen S, Parsons AJ, Bassett S, Christensen MJ, Hume DE, Johnson LJ, Johnson RD, Simpson WR, Stacke C, Voisey CR. High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytologist. 2007;173:787. doi: 10.1111/j.1469-8137.2006.01960.x. [DOI] [PubMed] [Google Scholar]
- 68.Wäli PR, Ahlholm JU, Helander M. Genetic structure of the systemic grass endophyte Epichloë festucae populations. Microbial Ecology. 2007;3:20. doi: 10.1007/s00248-006-9076-2. [DOI] [PubMed] [Google Scholar]