Abstract
In plants, mitogen-activated protein kinases (MAPK) have been implicated in signalling associated with many processes, including cellular differentiation, organ development, cell death and stress/hormone signalling. While MAPK cascades are known to act in the cytosol and the nucleus, sequence analysis of the Arabidopsis MAPK cascade proteins predicts the presence of import signals that would target some of them to other organelles. In vitro uptake experiments confirm the predicted import of an oxidant-responsive MAPKK, AtMKK4, into the chloroplast. Unexpectedly, the imported MKK4 protein was not processed through stromal peptidase-dependent cleavage of the N-terminal signal peptide, thus leaving the pre-protein intact. Nevertheless, the N-terminal region was shown to be essential both for the import process and for the ability of MKK4 to activate its cognate MAPK targets in vivo. MKK4 import also occurred irrespective of the activation status of the kinase. The import of this primarily cytosolic oxidant-stimulated AtMKK4 into the chloroplasts, organelles with high redox fluxes, suggests that one of the functions of MKK4 might be to help coordinate intercompartment responses to cellular redox imbalances.
Key words: cell death, chloroplast, compartmentation, MAPK, MAPK kinase, MPK6, MPK3, signal transduction, stroma, transit peptide
Introduction
In eukaryotic cells, MAPK (mitogen-activated protein kinase) circuits constitute an important signal integration network for multiple processes involved in maintaining cellular homeostasis. Canonical MAPK cascades are comprised of three hierarchically organized classes of protein kinases (MAPKs, MAPK kinases and MAPK kinase kinases) that transmit and amplify impinging signals, thereby leading to rapid and efficient activation of appropriate outputs. Arabidopsis thaliana possesses an array of 20 ERK-type MAPKs (AtMPK) that can be activated by a set of 10 upstream MAPK kinases (AtMKK); these, in turn, are activated by more than 60 MAPKK kinases (AtMKKK).1,2 Within this signaling matrix, one well-characterized activation module involves dual phosphorylation of AtMPK3 and AtMPK6 by AtMKK4/5, a process that is rapidly invoked by a wide range of environmental stresses, including wounding, pathogen attack, elicitor challenge and exposure to oxidants.3–6 Ectopic overexpression of MKK4 leads to increased disease resistance as well as hyper-activation of downstream MAPKs and eventual cell death,7,8 but how MKK4 itself is normally activated is unclear. It has been proposed that signals generated by increased cellular levels of reactive oxygen species (ROS) in Arabidopsis leaves can be sensed by a serine/threonine protein kinase, OXI 1,9 which then transmits the signal through an unidentified transduction cascade that ultimately activates AtMPK6 and AtMPK3.9 On the other hand, ANP1, an Arabidopsis MAPKKK, has also been reported to function as the upstream activator of MKK4 and MKK5 during oxidant-induced signaling.7,10
Compartmentalization of signaling proteins in eukaryotic cells allows them to provide specific functions within metabolically distinct micro-environments. Membrane-bound organelles such as chloroplasts, mitochondria and nuclei represent major cellular compartments that sequester and organize many individual proteins and whole pathway complexes, either temporarily or permanently. For example, mammalian casein kinase 2 (CK2) subunits have been identified in multiple sub-cellular compartments and appear to perform specialized functions in each.11 In plants, Arabidopsis homologs of CK2 subunits have similarly been found to localize to the nucleus, cytosol and chloroplasts.12 While it is generally assumed that MAPK modules operate in the cytosol, there is also some evidence for MAPK cascade components in other sub-cellular compartments. In mammalian cells, both nuclear and mitochondrial localization of ERKs have been observed,13,14 and while mammalian MEK1, which possesses an NES sequence, is usually located in the cytosol, rapid shuttling to the nucleus has also been observed.15 In Arabidopsis, AtMPK6 and three are reported to be translocated into the nucleus upon activation following ozone stress,6 while SIMK, the alfalfa orthologue of AtMPK6, was reported to be constitutively localized in the nucleus in root epidermal cells.16
Among the various organellar compartments in eukaryotic cells, chloroplasts are of particular interest, since they are unique to plants, provide critical metabolic functions and also represent a major locus of endogenous ROS production. Plastids participate in many different cellular processes, including maintenance of redox homeostasis, and must therefore communicate dynamically with other cellular compartments. In view of the rapid activation of plant MAPKs by oxidant stress and related signals, we asked whether any components of plant MAPK cascades might be located within the chloroplast compartment. Analysis of the amino acid sequences of all the MAPK, MAPKK and MAPKKK proteins annotated in the Arabidopsis genome revealed that several of these do possess predicted N-terminal chloroplastic transit peptides, including the ROS-responsive MAPKKs, MKK4 and MKK5 (Table 1). This observation prompted us to investigate whether import of MKK4 into the chloroplasts could be confirmed experimentally. In vitro uptake experiments demonstrated that the MKK4 protein is imported into plastids, but the N-terminal transit peptide is retained during the uptake process. Interestingly, the same N-terminal region of MKK4 that is required for plastid uptake is also essential for activation of the downstream MAPKs, MPK3 and MPK6.
Table 1.
Chloroplast target predictions for Arabidopsis MAPKK family members and putative MKK4 orthologues from other species
| MAPKK | Length (aa) | ChloroP Score | MAPKK | Length (aa) | ChloroP Score |
| ATMKK1 | 308 | 0.445 | ATMKK4 | 366 | 0.555-Y |
| ATMKK2 | 363 | 0.451 | NtMEK2 | 372 | 0.582-Y |
| ATMKK3 | 520 | 0.447 | SIMKK | 368 | 0.567-Y |
| ATMKK4 | 366 | 0.555-Y | PcMKK5 | 356 | 0.523-Y |
| ATMKK5 | 348 | 0.556-Y | StMKK4 | 374 | 0.582-Y |
| ATMKK6 | 356 | 0.460 | LeMKK2 | 359 | 0.586-Y |
| ATMKK7 | 307 | 0.477 | OsMKK4 | 369 | 0.552-Y |
| ATMKK8 | 293 | 0.554-Y | |||
| ATMKK9 | 310 | 0.496 | |||
| ATMKK10 | 305 | 0.472 |
aa, amino acids; Y denotes presence of a chloroplastic transit peptide as predicted by ChloroP. Protein sequences were analyzed using the ChloroP program.
Results
MKK4 and its orthologues possess a chloroplast transit peptide.
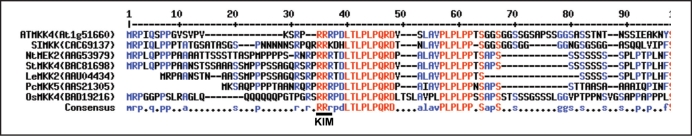
When the Arabidopsis MAPKK family was analyzed for the presence of chloroplast transit peptides using ChloroP (www.cbs.dtu.dk/services/ChloroP/), at least two members, MKK4 and MKK5, were predicted to contain a chloroplast transit sequence (Table 1). Both MKK4 and MKK5, which belong to the same clade of plant MAPKKs,2 are actively expressed MAPKKs (MIPS, AtGenExpress, S. Sritubtim and B. Ellis, unpublished results). Since at least partial functional redundancy of MKK4/MKK5 in various signaling processes has been previously established (refs. 7 and 22), we chose to characterize the possible import of MKK4 in more detail. Putative orthologues of AtMKK4 in all other eudicot species examined also possess a predicted plastid targeting signal at their N-terminus, a region which also contains the ‘kinase interacting motif’ (KIM; Fig. 1), indicating that both features are evolutionarily conserved.
Figure 1.
MKK4 and its putative orthologues possess a chloroplast transit signal at their N-terminus. Alignment of the N-terminus of AtMKK4 and its putative orthologues from Medicago (SIMKK), tobacco (NtMEK2), potato (StMKK4), tomato (LeMKK2), parsley (PcMKK5) and rice (OsMKK4); the MAPK-interacting motif (KIM) is underlined.
MKK4 is actively imported into pea chloroplasts.
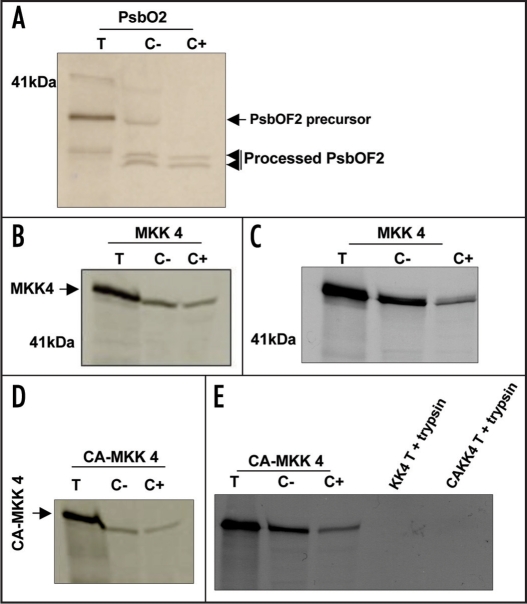
To test the functionality of the predicted uptake signal sequence, we initially examined the ability of purified chloroplasts to import MKK4 polypeptide. Control experiments confirmed that the pea chloroplast preparations could efficiently import and process the heterokont Heterosigma akashiwo nuclear-encoded thylakoid lumen protein, PsbO (also called OEC33 or ‘33-kDa oxygen-enhancer 1 protein’), which has been previously shown to be imported into pea chloroplasts19 (Fig. 2A). The imported PsbO was dually processed, with the removal of the stromal targeting domain followed by loss of the thylakoid lumen targeting domain, yielding fragments of two different masses (Fig. 2A). This result confirms the efficiency of the pea chloroplasts for import and processing of this protein. When the MKK4 protein was in vitro translated from AtMKK4 cDNA and the radiolabelled products (lane T) were incubated with isolated pea chloroplasts, SDS-PAGE fractionation of proteins recovered from the re-purified chloroplasts demonstrated the presence of the newly synthesized AtMKK4 protein (Fig. 2B and C). This association was unaffected either by treatment with thermolysin, which digests proteins non-specifically adsorbed to the chloroplast membrane (Fig. 2B), or with trypsin (Fig. 2C), which removes both, proteins non-specifically bound to the outer chloroplast membrane as well as proteins sequestered within the inter-membrane compartment. The partial reduction in the imported MKK4 protein observed following trypsin digestion of incubated chloroplasts may reflect tryptic removal of non-specifically bound MKK4 polypeptide within the inter-membrane compartment (Fig. 2C). Control experiments demonstrated that trypsin treatment of the in vitro translated MKK4 polypeptide resulted in its complete digestion (Fig. 2E). Taken together, these data show that the MKK4 import process directs the MKK4 polypeptide across both the outer and inner chloroplast membranes.
Figure 2.
MKK4 is imported into the stroma of pea chloroplasts irrespective of the activation status of the kinase. In vitro-translated PsbO, MKK4 and CA-MKK4 were incubated with intact pea chloroplasts. After incubation, samples were treated with either thermolysin (B and D) or trypsin (A, C and E), re-isolated, washed, lysed, fractionated and analyzed by SDS-PAGE followed by autoradiography. Translation products (lanes T); washed chloroplast fraction (lanes C-); protease-treated chloroplast fraction (lanes C+). (E) + trypsin lanes show SDS-PAGE fractionation of in vitro-translated MKK4 and CA-MKK4 digested with trypsin.
MKK4 Import is independent of the activation status of the kinase.
MAPKK function normally requires that the kinase domain be dually phosphorylated by upstream protein kinases such as MAPKK kinases. We therefore investigated whether such activation might alter the ability of the chloroplast to import MKK4.Site-directed mutagenesis was used to convert AtMKK4 to a structural analogue (T224D; S230E) of the phosphorylated form that displays constitutive catalytic activity25 and the ability of this ‘constitutively active’ derivative (CA-MKK4) to be imported into pea chloroplasts was tested. As with unmodified MKK4, in vitro translated CA-MKK4 polypeptide was also imported into purified chloroplasts (Fig. 2D and E). Trypsin treatment of in vitro-translated CA-MKK4 also resulted in complete digestion of CA-MKK4 (Fig. 2E). Thus, changes in the activation status of this MAPKK do not appear to be sufficient to control its subcellular compartmentation.
The MKK4 transit sequence is not removed during chloroplast import.
Most chloroplast proteins are encoded in the nuclear genome and must be synthesized on cytosolic ribosomes before being imported into the plastid compartment. This import process follows a well-characterized pathway that involves docking of the polypeptide at the import translocon, transport of the unfolded protein across both envelope membranes and proteolytic cleavage of the transit peptide in the stroma.26 Unexpectedly, however, the MKK4 polypeptide recovered from the chloroplasts retained the same electrophoretic mobility as the full-length in vitro -translated product, indicating that stromal processing peptidase (SPP)-dependent cleavage did not occur. This suggests that, following import into the chloroplasts, the putative transit sequence avoids processing by SPP.
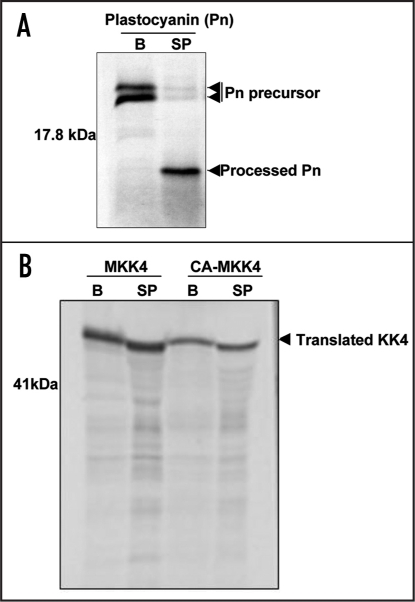
To test the ability of SPP to process MKK4 polypeptides, crude SPP was isolated from pea chloroplasts.21 The activity of this SPP preparation was tested by incubation with the full-length precursor of pea plastocyanin, a thylakoid lumenal protein,27 (Fig. 3A) which has been previously shown to be a substrate of SPP. The precursor of plastocyanin was readily processed to produce an intermediate form (only the transit peptide removed, Fig. 3A), but the same pea chloroplast SPP preparation was unable to process in vitro translated MKK4 or CA-MKK4 polypeptides (Fig. 3B), consistent with the MKK4 import pattern observed with intact chloroplasts.
Figure 3.
MKK4 is resistant to processing by pea stromal processing peptidase (SPP). (A) Radiolabelled pea plastocyanin precursor (accession number P16002) was incubated with either buffer alone or with a crude pea SPP preparation. (B) Similar assays were performed with in vitro-translated MKK4 and CA-MKK4 proteins. B, buffer only; SP, stromal peptidase.
The N-terminal chloroplast transit peptide has a dual function.
Recognition and processing of proteins imported into chloroplasts is generally considered to be driven by the structural properties of the polypeptide N-terminus. The failure of the MKK4 polypeptide to be processed by SPP despite its import into the stromal compartment suggested that this protein might possess an atypical transit peptide signal located elsewhere within the protein.28 To assess the contribution of the predicted MKK4 N-terminal import signal to MKK4 biological functions, we created a cDNA encoding a truncated version of CA-MKK4 that lacks the first 65 residues (ΔN65CA-MKK4). When this substrate was tested in our in vitro translation/pea chloroplast import assay, we observed no uptake of the truncated MKK4 (Fig. 4A), confirming that a chloroplast import signal is located in the N-terminal region of the protein, as originally predicted.
Figure 4.

The N-terminal transit sequence of MKK4 is required both for import and for activation of downstream MAPKs and associated cell death. (A) Radiolabelled ΔN65CA-MKK4 and Heterosigma PsbO precursors were incubated with intact pea chloroplasts. After incubation, samples were treated with trypsin, re-isolated, washed, lysed, fractionated and analyzed by SDS-PAGE, followed by autoradiography. Translation products (lanes T); washed chloroplast fraction (lanes C-); protease-treated chloroplast fraction (lanes C+). (B) Localized cell death induced by transient expression of CA-MKK4 and ΔN65CA-MKK4 in tobacco. (C) Downstream activation of SIPK and WIPK as detected by anti-pERK immunoblotting.
However, the N-terminal region of MAPKK proteins also typically contains a basic KIM which is thought to be essential for the association of MAPKKs with their target MAPKs.25 Deletion of this KIM region (NΔ64) from NtMEK2 (the tobacco orthologue of AtMKK4) was earlier found to block both activation of target MAPKs (SIPK and WIPK) and elicitation of cell death when a constitutively active version was transiently expressed in tobacco.29 We were able to confirm that transient expression of a CA-MKK4:FLAG construct in agroinfiltrated tobacco leaves rapidly activates the cytosolic target MAPKs, SIPK and WIPK (AtMPK6 and 3 orthologues) (Fig. 4C), as assessed by immunoblotting using an anti-phospho ERK antibody that detects phosphorylated forms of SIPK and WIPK. The ectopic expression of CA-MKK4:FLAG also led to death of the infiltrated tissue within 24 h (Fig. 4B). By contrast, expression of the ΔN65CA-MKK4 construct elicited neither effect (Fig. 4B and C).
Discussion
Non-canonical nature of MKK4 import.
Chloroplasts are major organelles for most plants since they perform photosynthesis as well as biosynthesis of a wide range of primary and secondary metabolites. However, while ∼3500–4000 proteins have been predicted to function in the chloroplasts,30 the plastid genome encodes only ∼200 genes, which makes polypeptide import an essential prerequisite to chloroplast-dependent metabolic and biosynthetic functions.30,31 Plant chloroplasts contain three distinct membranes (an outer and inner envelope, with a thylakoid membrane network harboring the photosynthetic complexes) and three soluble compartments (the inter-membrane space, the stroma and the thylakoid lumen), into which multiple proteins are imported from the cytosol.30 Proteins targeted to the inter-membrane space, inner envelope and stroma are generally thought to possess a cleavable N-terminal transit peptide, while the transit peptide signal of proteins targeted to the outer envelope is found internally in the mature protein and is not cleaved.30,31 Although the N-terminal transit sequences are highly divergent and have no common motifs,31 they are efficiently and precisely cleaved by the stromal processing peptidases.19,30
Our experiments show that, as predicted, the AtMKK4 polypeptide is imported into the chloroplast stroma in a process that is dependent on its putative N-terminal transit sequence. However, the N-terminus of the polypeptide was not processed either in the import studies or by isolated pea stromal peptidase preparations (Figs. 2 and 3). There have only been a few reports of nuclear-encoded chloroplast proteins that retain their transit sequence after import. A quinone oxidoreductase is targeted to the inner envelope by a transit sequence that was found to be essential for import but not removed in the stroma.28 Similarly, the chlorophyll a/b binding protein (CP29) of Chlamydomonas reinhardtii is targeted to the thylakoid but retains its transit peptide in the mature protein.32 However, in this case the N-terminal methionine was excised and the protein was acetylated at the new N-terminus, as well as being phosphorylated on the thr-6 residue. Interestingly, alignment of Chlamydomonas CP29 with its Arabidopsis orthologues (CP29.1, CP29.2) revealed that the mature Arabidopsis protein resulting from cleavage of the 31 aa N-terminal transit sequence aligns well at the N-terminus with the uncleaved Chlamydomonas CP29.32 The observation that Arabidopsis CP29.2 is also phosphorylated at thr-6 in the mature protein,33 provides further evidence for evolutionary conservation of this unusual import pattern. A somewhat more complicated example is the dual-targeted protein disulfide isomerase, RB60, of Chlamydomonas, whose N-terminal pre-sequence is cleaved upon import into the endoplasmic reticulum but retained upon import into the chloroplast.34 It is clear that N-terminal pre-sequences of organellar proteins can have more than one function, and are not all processed in the same way.
Dual function of the MKK4 N-terminus.
While the lack of processing of MKK4 at the N-terminus inside the chloroplast appears to be a unique and intriguing feature of this protein, this domain must also perform functions other than directing import into the plastid, since the N-terminally truncated ΔN65CA-MKK4 construct failed to activate WIPK/SIPK (MPK3/6) or to elicit a cell death response when expressed ectopically. All mammalian MAPKKs possess an N-terminal “D-domain,” which serves as the docking site for their MAPK substrates.35 The consensus D-domain structure (KIM) consists of a cluster of basic residues flanked C-terminally with hydrophobic residues [+++X (1–5) ϕXϕ; basic and hydrophobic residues are indicated by + and ϕ respectively], and a similar consensus has been observed in plant MKK sequences.29 Removal or alteration of the KIM region in NtMEK2, the tobacco orthologue of AtMKK4, resulted in loss of binding of NtMEK2 to its cognate MAPKs and of its ability to induce cell death.29 In mammalian MAPKKs, proteolytic cleavage of the N-terminus of MEKs by the anthrax lethal factor also results in the loss of MEK function due to impairment of binding to their respective downstream MAPKs.36
How the Arabidopsis MKK4 N-terminus avoids processing by the stromal peptidase is still not known, but the requirement of the N-terminus for substrate binding as well as import might indicate that, immediately after delivery into the chloroplast stroma, MKK4 substrates or interactors compete effectively with the SPP for binding to the kinase N-terminus. Our in vitro processing assays using crude SPP preparations (Fig. 3B) do not exclude this possibility, since MKK4 interactors could still be present in the crude SPP preparations,21 allowing them to interfere with the action of SPP. Alternatively, a conformational change in MKK4 or a failure of SPP to recognize a cleavable transit peptide could spare the MKK4 transit sequence.
Subcellular localization and possible role for MAPK cascade components.
Our evidence for import of AtMKK4, a redox-responsive MAPKK, into the chloroplast stroma raises the possibility that one or more MAPK pathways operate within the chloroplasts. Compartmentalization of MAPKs is known to influence their mediation of cellular responses in other eukaryotes.37,38 In plants, the Arabidopsis MAPKs, AtMPK6 and 3, were shown to translocate to the nucleus following their activation by ozone6 and the rice MAPK, BMWK1 (OsMKK17-1), was reported to localize to the nucleus and trigger phosphorylation-dependent enhancement of DNA binding activity of a transcription factor, OsEREBP1.39
Our results, along with previously published studies,8,29 point to a pro-cell death role for MKK4 in plants (Fig. 4), and differential sub-cellular localization of cell death-regulating proteins has been reported previously. For example, loss-of-function mutations in the Arabidopsis ACD2 (accelerated cell death2) gene result in uncontrolled expansion of pathogen-induced lesions on infected leaves,40 indicating that ACD2 plays an anti-apoptotic role in this context.41 The ACD2 protein (“red chlorophyll catabolite reductase”),40 localizes largely to the chloroplasts in healthy mature leaves but, following pathogen infection, it partitions into the chloroplasts, mitochondria and cytosol. MKK4 could conceivably undergo a similar partitioning in the course of its participation in oxidant/elicitor-induced cell death.
However, the biochemical function of MKK4 in the chloroplast stroma remains unknown. MKK proteins normally serve as activators of downstream MAPK targets, and organellar MAPK cascades have not been examined, although phosphorylated ERK1/2 has been detected within mammalian mitochondria.14 While there is no predicted chloroplast import signal in the N-terminus of either AtMPK3 or AtMPK6, which are the best-characterized targets of AtMKK4, the ability of MKK4 to activate other members of the MPK family in Arabidopsis remains largely unexplored. Our in silico analysis of predicted organelle uptake signals within the AtMPK family detected a strong transit signal in only one member, AtMPK8, but when the MPK8 cDNA was tested as an in vitro-translated import substrate with pea chloroplasts, no import was observed (data not shown). However, this does not preclude the possibility that another MAPK which is not predicted to localize in the chloroplast could serve in vivo as a substrate for MKK4 inside the chloroplast. Relying solely on prediction programs without experimental evidence can clearly be misleading, since a substantial proportion of the proteins recently detected in the plastid proteome by mass spectrometry were not predicted to reside in the chloroplast compartment.42 Likewise, while both MKK4 and MKK5 are predicted by ‘Target P’ to localize in the chloroplasts, only MKK4 is predicted to be targeted to the chloroplasts by ‘Wolf pSORT’ (http://wolfpsort.seq.cbrc.jp/) (data not shown). It is noteworthy that ectopic expression of an AtMKK4::GFP fusion protein in transgenic Arabidopsis was earlier reported to label discrete but uncharacterized sub-cytosolic components.43
The apparent lack of downstream MAPK target(s) for MKK4 in the plastid stroma could also mean that, in addition to its known role in activating MPK3/6 in the cytosol, MKK4 has other, as yet unidentified, non-MPK targets within the chloroplast. Comparative analysis of the stromal phospho-proteome of wild type and MKK4-transformed Arabidopsis plants following induction with DEX should help reveal potential stromal substrates of MKK4, and provide clues as to the role(s) of plastid-associated MKK4 in modulating plastid function.
Materials and Methods
Chloroplast target prediction for Arabidopsis MAPKK family members and putative MKK4 orthologues.
Protein sequences of the various Arabidopsis MAPKKs (AtMKK1-10) and putative AtMKK4 orthologues from other species were obtained from PlantsP (http://plantsp.genomics.purdue.edu/). These sequences were analyzed for the presence of a predicted chloroplast transit sequence using ChloroP, a neural network-based software package (www.cbs.dtu.dk/services/ChloroP/).17 A score >0.5 generated by the ChloroP algorithm predicts the presence of a chloroplast transit peptide (Y). For example, chloroplast-localized carbonic anhydrase from pea (accession AAA33652) has a network score of 0.594.
Arabidopsis MKK4 constructs, Agrobacterium-mediated transformation and chloroplast isolation.
Arabidopsis MKK4 cDNA was obtained through reverse transcription-PCR and cloned into pBlueScript vector using gene-specific primers. The constitutively active mutant CA-MKK4 was created through mutagenizing MKK4 via T224D and S230E changes, using a QuickChange site-directed mutagenesis kit (Stratagene) and confirmed by sequencing. The ΔN65CA-MKK4 (N-terminal deleted version of CA-MKK4) clone was created through PCR amplification and confirmed by sequencing. To create plant overexpression constructs, MKK4 or its mutated (CA-MKK4) or deletion construct (ΔN65CA-MKK4) were PCR-amplified from pBluescript and cloned into the steroid (dexamethasone)-inducible pTA7002 binary vector.18
In vitro transcription and translation.
In vitro transcription and translation were performed as (described previously in ref. 19). The various constructs were transcribed in vitro using the Cap-Scribe RNA polymerase kit (Roche Diagnostics, Canada), and translated with the Rabbit Reticulocyte Lysate System (Promega, USA) with Redivue L-[35S]-methionine (1000 Ci/mmol) (Amersham Pharmacia Biotech, UK). The reactions were terminated by adding 6X SDS sample buffer followed by analysis on a 15% SDS-PAGE gel and autoradiography.
Pea chloroplast import assays.
Eight- to ten-day-old pea seedlings (Pisum sativum var. Maestro) were used for isolation of intact chloroplasts, prepared as (described by Mould and Gray, ref. 20). Typically, import assays contained 50 µL of translation product, intact chloroplasts (0.5 mg chlorophyll/ml), 5 mM L-methionine and 10 mM MgATP in a final volume of 500 µL of import buffer (50 mM Hepes KOH, pH 8.0 and 0.33 M sorbitol), and were incubated in the light (100 µmol photons/m2/s) for 40 min at 24°C.20 After incubation, the chloroplasts were treated with either trypsin or thermolysin, re-isolated, washed, lysed and analyzed by SDS-PAGE followed by autoradiography.19
Preparation of pea SPP and processing assays.
Intact chloroplasts from pea seedlings were prepared as described above. An SPP preparation was isolated from intact pea chloroplasts as (described by Abad et al. ref. 21). Radiolabelled precursor protein (4 µl) was incubated with 40 µl SPP extract for 90 min at 27°C followed by analysis through SDS-PAGE and autoradiography.
Agrobacterium-mediated transient transformation and analysis.
Twenty-four hours following infiltration of leaves of six- to eight-week-old tobacco plants, with Agrobacterium tumefaciens GV3101 harboring the various DEX-inducible constructs (CA-MKK4 or ΔN65CA-MKK4), the infiltrated zones were treated with 30 µM DEX.22 The infiltrated zones were harvested at 4 and 8 h following DEX treatment, for protein extraction to assay MAPK activation through Western blotting (see below), or were scored for lesion formation after 24–48 h.
Protein extraction and Western blotting.
Total protein extracts (40–80 µg) from the CA-MKK4- and ΔN65CA-MKK4-infiltrated zones were prepared and used for western blotting with polyclonal phospho-specific anti-MAPK (anti-pERK, p44/42) antibodies (New England Biolabs Inc., Beverly, MA, USA), as (described earlier ref. 23). The CA-MKK4-activated MAPKs in tobacco have been previously identified as SIPK and WIPK through immunoprecipitation with SIPK- and WIPK-specific antibodies.24 The anti-pERK antibody employed in the present study was previously shown to detect phosphorylated forms of SIPK and WIPK in ozone-exposed WT tobacco tissue, as well as phosphorylated SIPK (pSIPK) in SIPK-overexpressing tissue.24
Acknowledgements
We thank Dr. N.-H. Chua (Rockefeller University, New York) for the pTA7002 vector. The full-length clone of the pea plastocyanin (accession number P16002) was kindly provided by Dr. S.M. Theg. Funding for this research was provided to BG and BE by the Natural Sciences and Engineering Research Council of Canada.
Abbreviations
- MAPK
mitogen-activated protein kinase
- MKK
MAPK kinase
- ROS
reactive oxygen species
- SPP
stromal processing peptidase
- DEX
dexamethasone
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/4856
References
- 1.Hamel LP, Nicole MC, Sritubtim S, Morency MJ, Ellis M, Ehlting J, Beaudoin N, Barbazuk B, Klessig D, Lee J, Martin G, Mundy J, Ohashi Y, Scheel D, Sheen J, Xing T, Zhang S, Seguin A, Ellis B. Ancient signals: Comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci. 2006;11:1928. doi: 10.1016/j.tplants.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Ichimura, et al. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002;7:301–308. doi: 10.1016/s1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- 3.He P, Shan L, Lin N, Martin G, Kemmerling B, Nurnberger T, Sheen J. Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell. 2006;125:563–575. doi: 10.1016/j.cell.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 4.Nuhse T, Peck S, Hirt H, Boller T. Microbial elicitors induce activation and dual phosphorylation of the Arabidopsis thaliana MAPK 6. J Biol Chem. 2000;275:7521–7526. doi: 10.1074/jbc.275.11.7521. [DOI] [PubMed] [Google Scholar]
- 5.Bogre L, Ligterink W, Meskiene I, Barker P, Heberle-Bors E, Huskisson N, Hirt H. Wounding induces the rapid and transient activation of a specific MAP kinase pathway. Plant Cell. 1997;9:75–83. doi: 10.1105/tpc.9.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahlfors R, Macioszek V, Rudd J, Brosche M, Schlichting R, Scheel D, Kangasjarvi J. Wounding induces the rapid and transient activation of a specific MAP kinase pathway. Plant J. 2004;40:512–522. doi: 10.1111/j.1365-313X.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- 7.Asai T, Tena G, Plotnikova J, Willmann M, Chiu W, Gomez-Gomez L, Boller T, Ausubel F, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Jin H, Kim C, Baker B, Zhang S. Interaction between two mitogen-activated protein kinases during tobacco defense signaling. Plant J. 2003;34:149–160. doi: 10.1046/j.1365-313x.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- 9.Rentel M, Lecourieux D, Ouaked F, Usher S, Petersen L, Okamoto H, Knight H, Peck S, Grierson C, Hirt H, Knight M. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature. 2004;427:858–861. doi: 10.1038/nature02353. [DOI] [PubMed] [Google Scholar]
- 10.Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faust M, Montenarh M. Subcellular localization of protein kinase CK2 A key to its function? Cell Tissue Res. 2000;301:329–340. doi: 10.1007/s004410000256. [DOI] [PubMed] [Google Scholar]
- 12.Salinas P, Fuentes D, Vidal E, Jordana X, Echeverria M, Holuigue L. An extensive survey of CK2 α and β subunits in Arabidopsis: Multiple isoforms exhibit differential subcellular localization. Plant Cell Physiol. 2006;47:1295–1308. doi: 10.1093/pcp/pcj100. [DOI] [PubMed] [Google Scholar]
- 13.Cobb M, Goldsmith E. Dimerization in MAP-kinase signaling. Trends Biochem Sci. 2000;25:7–9. doi: 10.1016/s0968-0004(99)01508-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, Guo F, Shelburne J, Watkins S, Chu C. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003;3:473–481. doi: 10.1111/j.1750-3639.2003.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaaro H, Rubinfeld H, Hanoch T, Seger R. Nuclear translocation of mitogen-activated protein kinase kinase MEK1 in response to mitogenic stimulation. Proc Natl Acad Sci USA. 1997;94:3742–3747. doi: 10.1073/pnas.94.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samaj J, Ovecka M, Hlavacka A, Lecourieux F, Meskiene I, Lichtscheidl I, Lenart P, Salaj J, Volkmann D, Bogre L, Baluska F, Hirt H. Involvement of the mitogen-activated protein kinase SIMK in regulation of root hair tip growth. EMBO J. 2002;21:3296–3306. doi: 10.1093/emboj/cdf349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emanuelsson O, Nielsen H, von Heijne G. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci. 1999;8:978–984. doi: 10.1110/ps.8.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoyama T, Chua NH. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 1997;11:605–612. doi: 10.1046/j.1365-313x.1997.11030605.x. [DOI] [PubMed] [Google Scholar]
- 19.Chaal B, Green B. Protein import pathways in ‘complex’ chloroplasts derived from secondary endosymbiosis involving a red algal ancestor. Plant Mol Biol. 2005;53:333–342. doi: 10.1007/s11103-004-7848-y. [DOI] [PubMed] [Google Scholar]
- 20.Mould R, Gray J. Preparation of chloroplasts for protein synthesis and protein import. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. New York: Academic Press; 1998. pp. 81–86.pp. 286–292. [Google Scholar]
- 21.Abad M, Clark S, Lamppa G. Properties of a chloroplast enzyme that cleaves the chlorophyll a/b binding protein precursor: Optimization of an organelle-free reaction. Plant Physiol. 1989;90:117–124. doi: 10.1104/pp.90.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren D, Yang H, Zhang S. Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem. 2002;277:559–565. doi: 10.1074/jbc.M109495200. [DOI] [PubMed] [Google Scholar]
- 23.Samuel M, Ellis B. Double jeopardy: Both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. The Plant Cell. 2002;14:2059–2069. doi: 10.1105/tpc.002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuel M, Miles G, Ellis B. Ozone treatment rapidly activates MAP kinase signalling in plants. Plant J. 2000;22:367–376. doi: 10.1046/j.1365-313x.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- 25.Grewal S, Molina D, Bardwell L. Mitogen-activated protein kinase MAPK-docking sites in MAPK kinases function as tethers that are crucial for MAPK regulation in vivo. Cell Signalling. 2006;18:123–134. doi: 10.1016/j.cellsig.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis P, Robinson C. Mechanisms of protein import and routing in chloroplasts. Curr Biol. 2004;14:1064–1077. doi: 10.1016/j.cub.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 27.Cline K, Ettinger W, Theg S. Protein-specific energy requirements for protein transport across or into thylakoid membranes Two lumenal proteins are transported in the absence of ATP. J Biol Chem. 1992;267:2688–2696. [PubMed] [Google Scholar]
- 28.Miras S, Salvi D, Ferro M, Grunwald D, Garin J, Joyard J, Rolland N. Non-canonical transit peptide for import into the chloroplast. J Biol Chem. 2002;277:47770–47778. doi: 10.1074/jbc.M207477200. [DOI] [PubMed] [Google Scholar]
- 29.Jin H, Liu Y, Yang KY, Kim C, Baker B, Zhang S. Function of mitogen-activated protein kinase pathway in N gene-mediated resistance in tobacco. Plant J. 2003;33:719–731. doi: 10.1046/j.1365-313x.2003.01664.x. [DOI] [PubMed] [Google Scholar]
- 30.Soll J, Schleiff E. Protein import into chloroplasts. Nat Rev Mol Cell Biol. 2004;5:198–208. doi: 10.1038/nrm1333. [DOI] [PubMed] [Google Scholar]
- 31.Bruce B. The paradox of plastid transit peptides: Conservation of function despite divergence in primary structure. Biochim Biophys Acta. 2001;1541:2–21. doi: 10.1016/s0167-4889(01)00149-5. [DOI] [PubMed] [Google Scholar]
- 32.Turkina M, Villarejo A, Vener A. The transit peptide of CP29 thylakoid protein in Chlamydomonas reinhardtii is not removed but undergoes acetylation and phosphorylation. FEBS Lett. 2004;564:104–108. doi: 10.1016/S0014-5793(04)00323-0. [DOI] [PubMed] [Google Scholar]
- 33.Hansson M, Vener A. Identification of three previously unknown in vivo protein phosphorylation sites in thylakoid membranes of Arabidopsis thaliana. Mol Cell Proteomics. 2003;2:550–559. doi: 10.1074/mcp.M300050-MCP200. [DOI] [PubMed] [Google Scholar]
- 34.Levitan A, Trebitsh T, Kiss V, Pereg Y, Dangoor I, Danon A. Dual targeting of the protein disulfide isomerase RB60 to the chloroplast and the endoplasmic reticulum. Proc Natl Acad Sci USA. 2005;102:6225–6230. doi: 10.1073/pnas.0500676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanoue T, Nishida E. Docking interactions in the mitogen-activated protein kinase cascades. Pharmacol Ther. 2002;93:193–202. doi: 10.1016/s0163-7258(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 36.Bardwell A, Abdollahi M, Bardwell L. Anthrax lethal factor-cleavage products of MAPK (mitogen-activated protein kinase) kinases exhibit reduced binding to their cognate MAPKs. Biochem J. 2004;378:569–577. doi: 10.1042/BJ20031382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu C, Levinthal D, Kulich S, Chalovich E, DeFranco D. Oxidative neuronal injury: The dark side of ERK1/2. Eur J Biochem. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pouyssegur J, Lenormand P. Fidelity and spatio-temporal control in MAP kinase ERKs signaling. Eur J Biochem. 2003;270:3291–3299. doi: 10.1046/j.1432-1033.2003.03707.x. [DOI] [PubMed] [Google Scholar]
- 39.Cheong Y, Moon B, Kim J, Kim C, Kim M, Kim I, Park C, Kim J, Park B, Koo S, Yoon H, Chung W, Lim C, Lee S, Cho M. BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol. 2003;132:1961–1972. doi: 10.1104/pp.103.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mach J, Castillo A, Hoogstraten R, Greenberg J. The Arabidopsis accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc Natl Acad Sci USA. 2001;98:771–776. doi: 10.1073/pnas.021465298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao N, Greenberg J. Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. The Plant Cell. 2006;18:397–411. doi: 10.1105/tpc.105.036251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleffmann T, Russenberger D, von Zychlinski A, Christopher W, Sjolander K, Gruissem W, Baginsky S. The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr Biol. 2004;14:354–362. doi: 10.1016/j.cub.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 43.Koroleva O, Tomlinson M, Leader D, Shaw P, Doonan J. High-throughput protein localization in Arabidopsis using Agrobacterium-mediated transient expression of GFP-ORF fusions. Plant J. 2005;41:162–174. doi: 10.1111/j.1365-313X.2004.02281.x. [DOI] [PubMed] [Google Scholar]