Abstract
The orchid genera Catasetum employs a hair-trigger activated, pollen release mechanism, which forcibly attaches pollen sacs onto foraging insects in the New World tropics. This remarkable adaptation was studied extensively by Charles Darwin and he termed this rapid response “sensitiveness.” Using high speed video cameras with a frame speed of 1000 fps, this rapid release was filmed and from the subsequent footage, velocity, speed, acceleration, force and kinetic energy were computed.
Key words: Catasetum, pollination, Darwin, high-speed photography
I have reserved for separate description one sub-family of the Vandeæ, namely the Catasetidæ, which may, I think, be considered as the most remarkable of all Orchids.”
Charles Darwin, 1862
Introduction
Rapid touch responses in plants occur in a relatively small number of species. The most familiar examples are the Venus flytrap (Dionaea muscipula) and the sensitive plant (Mimosa pudica). Recent reviews of touch responses in various plant species are offered by Braam1 and McCormack.2 It is only recently that high speed videography has been utilized to capture the most rapid of these movements with image capture rates at high as 10,000 frames per second being utilized to measure plant movements as rapid as 3.1 m/s in the pollen launch of Cornus canadensis.3
The family Orchidaceae is one of the two largest families of angiosperms, with over 750 genera and over 19,000 species. These are spread worldwide, with species on every continent except Antarctica. Flowers are nearly always bisexual, with flowers borne singly or in spikes, racemes, or panicles. Observed pollinators include bees, wasps, flies, ants, beetles, hummingbirds, bats and frogs.4
The genus Catasetum (from the Greek katá meaning downward, and the Latin seta meaning bristle) ranges from the West Indies and Mexico, south to Argentina. They are characterized by a fleshy pseudobulb with eight to twelve leaves with inflorescences that arise from the base of the pseudobulb. These inflorescences bear either female or male flowers and exhibits the condition known as sexual dimorphism, as the flowers exhibit very different shapes depending upon the sex, a condition that is highly unusual in the orchid family. The male flowers are usually colorful and display a remarkable pollen ejection mechanism. Protruding from the rostellum (the often beak like sterile third stigma lying between the functional stigmas and stamen) is a pair of antennae, which when touched responds to stimulation by ejecting a two part projectile.5
The outermost component is a protective sheath (the anther) that covers the actual pollen sacs. The tip of this sheath is attached to the tip of the rostellum. The second component, the pollinium, has three structures. The viscidium, a disc of 3 mm, contains a sticky matter that adheres to the back of the pollinator. The viscidium is attached to a short stipe, upon which two pollen sacs are located. Its structure is analogous to an arrow; with the viscidium as the flint and the pollen sacs as the fletching. When launched, the viscidium is the forward most component of the assemblage and is the first to make contact with an insect, adhering to the startled pollinator's back, perfectly positioned for pollination when subsequent female flowers are visited.
The orchid of our trials was Catasetum fimbriatum Lindl. An epiphytic species native to southern tropical South America, it is often found growing on trunks of palm trees with inflorescences bearing 7 to 15 fleshy, musky smelling flowers.
The opening quote is taken from Darwin's book On the Various Contrivances by Which British and Foreign Orchids are Fertilised by Insects.6 The book is a treatise on various pollinators of orchids and details the unique method of pollination of the Catasetidae. Darwin analyzed the structure of orchid flowers and how this related to pollination. It is of no surprise that he designated an entire chapter to orchids that forcefully eject their pollen. For the most part he focused on Catasetum, going into great detail about the flower; naming and describing its various structures. In one passage he described the trigger mechanism:
That in Catasetum the rostellum is prolonged into two curved tapering horns, or, as I shall call them, antennae, which stand over the labellum where insects alight, and the excitement of a touch is conveyed along these antennae to the membrane which has to be ruptured.
Darwin recognized a mechanical response to stimulation, applying the term “sensitiveness” for this reaction. He noted that it was probably turgor pressure that caused the pollinium's ejection. A rupturing of a certain membrane releases the pollinium from the flower where it springs onto an unsuspecting insect's back. Darwin addressed the fact that the flower is essentially sensing the stimulation, applying the term “sensitiveness” for this reaction and tested the degree of sensitivity in various genera of Catasteum.
In C. tridentatum a stream of air and of cold water from a small pipe did not suffice; nor in any case did a touch from a human hair; so that the antennae are less sensitive than the rostellum of Listera. Such extreme sensitiveness would indeed have been useless to the plant, for we have reason to believe that the flowers are visited by bulky insects.
It is worth noting that all specimens observed by Darwin came to him from various British nurseries and private collectors.7 Darwin may have observed Catasetum and other orchids in the wild during the voyage of the Beagle but makes no mention of any orchid in his account of this journey. It has now been confirmed that Catasetum species are indeed pollinated by large Euglossid bees, affirming the brilliance of Darwin's observational genius.
Filming of the pollinium's ejection was first accomplished by Ebel et al. in 1974 using a high speed film camera. Speed and impulse of the pollinium were factored from their footage.8
Like Darwin, we also hoped to observe the unique method of pollination found in C. fimbriatum. We sought to examine the structures and their importance in pollination. However, unlike Darwin we had the aide of advanced photographic techniques. By using high-speed video photography, we were able to record the motion of the pollinium during ejection, analyze the footage and quantify the kinetics of the pollinium's motion over time.
Results
Successful capture of the pollinium launch was only achieved with one flower and this precludes statistical analysis. These data therefore only present a preliminary study but points to the importance of Catasetum as a study plant for rapid motion research in plants.
We measured the pollinium's motion over a period of 22 milliseconds. Unfortunately, due to the structure of the flower, we were not able to observe the pollinium between its initial point of movement and millisecond 1. We could however observe and calculate the pollinium's kinematics from millisecond 2 onward. Future trials with a higher rate of image capture (fps) may allow for finer quantification.
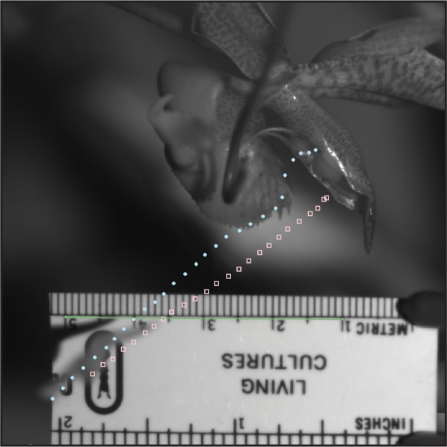
The motion of the combined pollinium and anther is complex as the viscidium is translating and rotating. The center of mass of the whole structure appears to move initially in a straight line downward and to the left. This is an approximation though, since the whole structure is actually rotating with the bottom end acting as a hinge. A few miliseconds after the top is released, the orchid pushes on the whole structure at the contact point at the bottom, which reduces the rotation, but does not stop the rotation. This complicates data analysis as the kinematics are vastly different for the viscidium itself, versus an estimated center of mass point closer to the middle of the assemblage. We choose viscidium as the data collection point for maximum velocity and determined also the maximum acceleration for this point. Figures for force and kinetic energy were derived using the maximum velocity and acceleration figures for the estimated center of mass as the data collection point.
Velocity and speed.
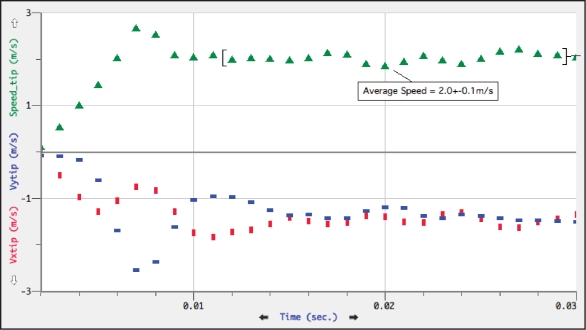
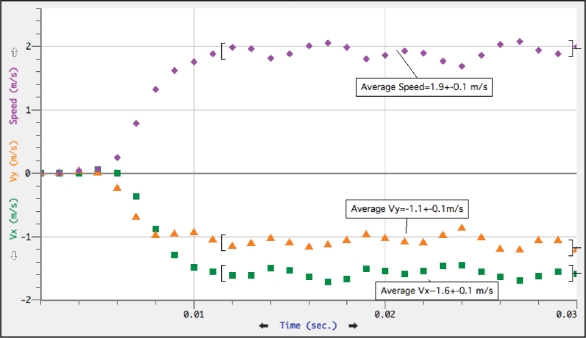
The distance that both the viscidium and estimated center of mass traveled per picture frame (one millisecond) was measured using Logger Pro 3 after having established a distance scale from the plastic ruler within the picture frame. Once all data points were entered, velocity, speed and acceleration could be derived. The largest distance traveled the viscidium traveled in a single millisecond was 2.65 mm. At this point the speed of the viscidium was 2.65 m/s (9.9 km/h) (Fig. 3). Maximum speed for the center of mass was 2.10 m/s (Fig. 4).
Figure 3.
Viscidium velocity and speed at one millisecond intervals. Velocity (red dots, x coordinate; blue dots, y coordinate) and speed (green triangles).
Figure 4.
Center of mass velocity and speed at one millisecond intervals. Velocity (green squares; x coordinate, orange triangles; y coordinate) and speed (purple diamonds).
By way of comparison, this compares favorably to the velocity of the head of a Pit Viper during striking, measured at 1.62 m/s (5.83 km/h) Vincent et al.9
Acceleration.
The viscidium could not be observed at resting stage as it was obscured by the anther and rostellum. Its initial thrust could therefore not be observed with certainty and acceleration could not be measured until the pollinium and anther actually were observed to be in motion, with the viscidium already at some undetermined velocity. It may be that greater velocity occurred earlier but could not be measured as for reasons stated. Using the measurements for velocity for this time period yielded a figure of 925m/s2 as the maximum acceleration between two data points.
By way of comparison, the maximum acceleration of the head of a Pit Viper during striking has been measured at 75.5 m/s2.9 However our figure for acceleration was far less than the 24,000m/s2 recorded by Edwards et al. for the accelerating stamens of Cornus canadensis.3
Force.
In order to calculate the force the pollinium was exerting it was necessary to find its weight. In our footage, as the pollinium tears away from the rostellum it carries with it the shield-like anther. Upon impact upon a pollinator, these two components separate but force in flight and upon impact would be based upon the combined weight of these two structures and we used the estimated center of mass as the data point from which we derived acceleration figures. The pollinium weighed 0.02 g and the anther weighed 0.03 g, giving a total weight of 0.05 g. The force produced by this mass at the point of maximum measurable acceleration was calculated by multiplying mass times acceleration and yielded a value of 2.90 x 10-2 Newtons.
Kinetic energy.
The kinetic energy of the pollinium and anther was calculated by multiplying one-half the mass times the velocity (highest figure for the center of mass data point) squared and resulted in a figure of 90 x 10-6 Joules.
Discussion
The mechanical response of Catasetum to stimuli is amazingly rapid for an organism with no nerves or muscle systems. Simons in his book The Action Plant, discusses Catasetum pollination and gives a figure of 300 cm/sec (10.8 km/hr) for the speed of the pollinium but gives no citation for this figure.10 The highest speed for Catasetum fimbriatum, as measured by Ebel et al. was 3.03 m/s with a mean of 2.76 m/s over nine trials.8 Our measurement of 2.65 m/s reflects an amazing consistency given that we were working with different clones in different laboratories. These measurements show Catasetum to be capable of some of the fastest velocities, speeds and accelerations within the plant kingdom and the speed compares favorably to the figure of 3.1 m/s given for the stamens of Cornus canadensis as measured by Edwards et al.3
The mechanism for communicating the stimuli signal within Catasetum is still to be determined but the rapid response of genera such as Dionea has been suggested by Trebacz et al. to be due to an action potential involving CA2+, Cl- and K+.9
A fertile course of inquiry would be to accurately record the moment of contact between a probe and the antennae, the moment of eruption and factor the speed of the signal through the antennae to the area from which the pollinium emerges.
The rapid mechanical placement of pollen upon a visiting pollinator as a response to the touch stimuli of this pollinator may be unique to the Orchid family with Catasetum, Mormodes and Cycnoches being the only genera so far known to exhibit this adaptation.
The structure of pollination mechanism has evolved for optimum placement of the pollinium on visiting foraging insects, ensuring outcrossing with female flowers. However, inactive anthers in other plant species are successful in pollen placement on foraging pollinators so the advantage may be in the training aspects of the rapid force of the Catasetum's pollinium.
Romero and Nelson observed that the impacted bees would not return to male flowers due to the forceful and surprising emplacement of the pollen payload received at the first male flower.12
This behavior modification may have the effect of reducing competition for fertile female flowers. The velocity and force of the pollinium ensures rapid attachment but avoids permanent or lethal damage to the unsuspecting pollinator and thereafter cultivates a tendency of male flower avoidance.
Jensen reported on C. maculatum in Costa Rica and observed that male flowers rapidly attract bees due to an early morning release of odor, and that within 45 minutes, five flowers had successfully attached five pollinia to five bees. “Virtually every bee that leaves a male flower with a pollinium pollinates a flower, if this is so, a male C. maculatum has the highest pollen-donation success rate of any obligatory outcrossing plant known.”13
Dodson's field work showed that a plant tends to produce a male or female inflorescences based upon ecological factors; primarily light levels and secondarily moisture levels, with male inflorescences resulting from lower light levels.14
Catasetum therefore has the ability to alter its sexual expression based on environmental cues or disruptions such as a new canopy gap. Male flowers rapidly attract and forcibly enlist bees as pollen envoys and train the same bees to avoid other males.
With over a hundred described species of Catasetum, and many horticultural hybrids, higher figures for the kinematics of the genus are indeed possible. The internet has made locating plants from commercial sources and botanical gardens far easier than in past decades and as the costs for high end technology decreases, Catasetum, Cycnoches, and Mormodes provide over 200 species which would be prime candidates for research into touch sensitive movements and plant speed trials (botanical kinematics).
Footage of our Catasetum trial can be viewed and downloaded at www.smith.edu/garden/Newsletter/spr2007/orchid-video.html.
Materials and Methods
Plants.
A mature flowering specimen of Catasetum fimbriatum, obtained from Smith College Botanic Garden (Accession #330-01).
Camera.
Vision Research Phantom V5.0 high speed digital imaging system, recording at a rate of 1,000 pps (pictures per second), equipped with Elicar 90-mm focal-length Macro, model V-HQ lens. Lighting was provided by a single halogen lamp.
Programs utilized for viewing and analysis: Phantom Cineviewer 606. Vernier Logger Pro 3.
Where.
The high speed imaging lab at the Edgerton Center, Massachusetts Institute of Technology, Cambridge, Massachusetts, December 2004.
Method.
Ambient temperature was 24°C. Flowers were removed of any petals that would obstruct viewing and photography. Flowers were positioned approximately three feet from camera with a scale of measure placed beneath the flower in viewing range. The Phanton V5.0 camera was equipped with a macro lens to allow greater range of focus and exposure and set to record at 1,000 frames per second. A halogen lamp was used to provide lighting for the camera. The camera was activated and the orchid's antennae were stimulated by a small wire until the pollinium was ejected. Picture sequence was analyzed using the viewing program Cineviewer 606 and Logger Pro 3 was used to log positions of the pollinium and anther during launch and to determine kinematic data. These programs allowed for frame by frame observation of the pollinium's trajectory through the field of view. The Derivative Savitsky-Golay function in Loggerpro 3 was used to calculate the derivative, with seven points utilized in the S-G part of the calculation.
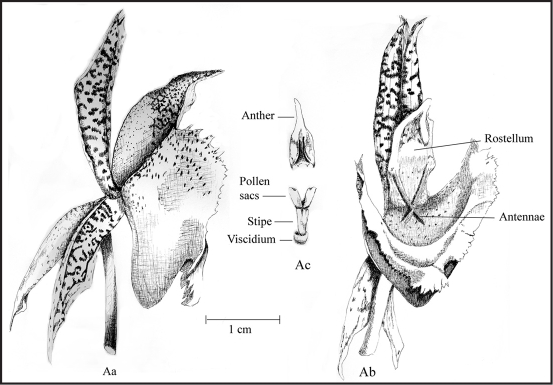
Figure 1.
Catasetum fimbriatum. Aa, side view of single flower; Ab, Face view; Ac, anther and pollinium showing pollen sacs, stipe and viscidium.
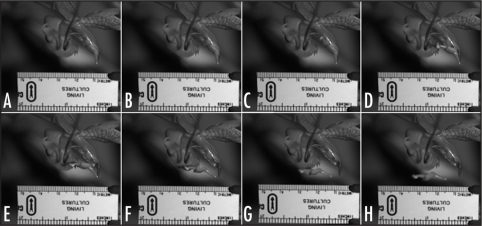
Figure 2.
A 16 millisecond sequence of still frames selected at 2 millisecond intervals, illustrates the flower at rest (A), the initial appearance of the viscidium (B), and the subsequent launch of the pollinium (C–H).
Figure 5.
Data collection points for the viscidium (solid dots) and the estimated center of mass (open dots).
Acknowledgements
We wish to thank Dr. Wilford Neptune for his kindly donation of orchid specimens to the Smith College Botanic Garden, including the above mentioned Catasetum. We also wish to thank Pamela A. Dods for her rendition of the Catasetum flower.
Note
Color figures can be found at: http://www.landesbioscience.com/journals/psb/article/4980
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/4980
References
- 1.Braam J. In touch: Plant responses to mechanical stimuli. New Phytologist. 2005;165:373–395. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 2.McCormack E, Velasquez L, Delk N, Braam J. Touch-responsive behaviour and gene expression in plants. In: Baluška F, Mancuso S, Volkmann D, editors. Communication in Plants, Neuronal Aspects of Life. Berlin Heidelberg: Springer-Verlag; 2006. pp. 249–260. [Google Scholar]
- 3.Edwards J, Whitaker D, Klionsky S, Laskowski M. A record breaking pollen catapult. Nature. 2005;435:164. doi: 10.1038/435164a. [DOI] [PubMed] [Google Scholar]
- 4.Heywood V. Flowering Plants of the World. London, UK: B.T. Batsford Ltd.; 1993. Orchidaceae. [Google Scholar]
- 5.Bechtel H, Cribb P, Launert E. The Manual of Cultivated Orchid Species. Cambridge, MA: MIT Press; 1981. [Google Scholar]
- 6.Darwin C. On the Various Contrivances by which British and Foreign Orchids are Fertilised by Insects. London, UK: John Murray; 1862. Catasetidæ, the most remarkable of all orchids; pp. 211–285. [Google Scholar]
- 7.Heriz-Smith S. James veitch and sons of exeter and chelsea, 1853–1870. Garden History. 1989;17:135–153. [Google Scholar]
- 8.Ebel F, Hagen A, Puppe K, Roth HJ, Roth J. Beobachtungen über das bewegungsverhalten des pollinariums von catasetum fimbriatum lindl. während abschuß, flug und landung. Flora Bd. 1974;163:342–356. [Google Scholar]
- 9.Vincent S, Herrel A, Irschick D. Comparison of aquatic versus terrestrial strikes in the pitviper, agkistrodon piscivorus. J Exp Zool. 2005;303:476–488. doi: 10.1002/jez.a.179. [DOI] [PubMed] [Google Scholar]
- 10.Simons P. The Action Plant, Movement and Nervous Behavior in Plants. Oxford: Blackwell; 1992. [Google Scholar]
- 11.Trebacz K, Dziubinska H, Krol E. Electric signals in long distance communication in plants. In: Baluška F, Mancuso S, Volkmann D, editors. Communication in Plants, Neuronal Aspects of Life. Berlin Heidelberg: Springer -Verlag; 2006. pp. 277–290. [Google Scholar]
- 12.Romero G, Nelson C. Sexual dimorphism in catasetum orchids: Forcible pollen emplacement and male flower competition. Science. 1986;232:1538–1540. doi: 10.1126/science.232.4757.1538. [DOI] [PubMed] [Google Scholar]
- 13.Janzen D. Differential visitation of catasetum orchid male and female flowers. Biotropica. 1981;13:77. [Google Scholar]
- 14.Dodson C. Pollination and variation in the subtribe catasetinae (orchidaceae) Ann Mis Bot Gar. 1962;49:35–56. [Google Scholar]