Abstract
We showed previously that grafting transmitted silencing occurred when transgenic ACC oxidase 1 (ACO1) overexpressing tomato plants that also produced siRNAs were grafted onto transgenic stocks that already showed strong silencing. The presence of siRNAs in these overexpressing scions may indicate that silencing, though inefficient, may already occur at a low level before grafting. To test if a silencing state with a relatively high level of target mRNA can be shifted towards further more effective silencing, we grafted an ACO1 antisense (AS) line with a high level of antisense ACO1 transgene mRNA and low level of siRNAs to the ACO1 strong silencer stock. The AS mRNA level was reduced dramatically two weeks after grafting. More interestingly, self-grafting of ACO1 overexpressers and AS lines also induced strong silencing in the scions. We suggest that grafting transmitted silencing may involve the switching from an inefficient or weak silencing state to a stronger silencing by a systemic silencing signal, similar to the change of silencing states that sometimes occurs during development. Control experiments using non-transgenic stocks designed to test whether wounding alone is responsible for generating a signal that enhances silencing in transgenic scions gave negative results. We propose that the build-up of silencing signal and/or molecules at both sides of the grafting junction and their sudden release when the phloem is reconnected may be critical to grafting transmitted silencing.
Key words: posttranscriptional gene silencing, siRNA, grafting, systemic silencing signal
It is well established that silencing can be transmitted to a non-silencing scion by grafting onto a silencing stock, provided that a high level of target mRNA is accumulated in the scion before grafting.1,2 Silencing can also be transmitted from a scion to a stock. The transmission direction can be manipulated by changing the source and sink relationship, suggesting transmission through phloem.3 In most cases tested, the scion contains the same transgene as in the stock and the high level of target mRNA is largely due to the accumulation of mRNAs from the transgene by transcription from a strong promoter such as the CaMV 35S promoter. We have shown recently that grafting-transmitted silencing occurs when ACC oxidase 1 (ACO1) overexpressers are grafted to a strong (ACO1) silencer rootstock in tomato.4 Interestingly, small interfering RNAs (siRNAs) could be detected in the overexpressers before grafting. Since different versions of the silencing transgene were available, the effect of transgenes in the stock and scion could be distinguished. This provided us with an excellent system to investigate transmission of the systemic silencing signal. Using the different transgene constructs, we were able to show that the siRNAs arise from the 3′ region of the transgene in the overexpressers (scion) and the 5′ region in the strong silencer (stock)4,5 (Fig. 1). This showed that the grafting process did not change the siRNA generation site to conform to that in the silencer stock but stimulated the production or amplification of the existing siRNAs in the scion. We proposed that another signal, such as larger aberrant RNAs (abRNA), rather than siRNAs, might be the systemic silencing signal. This grafting-transmitted silencing is maintained even in plants propagated from cuttings of the scions (data not shown), similar to the maintenance of silencing after scions with grafting-transmitted silencing are regrafted onto non-silencing stocks.6 Silencing is not inherited, however, since the grafting-transmitted silencing is not maintained in progenies.6
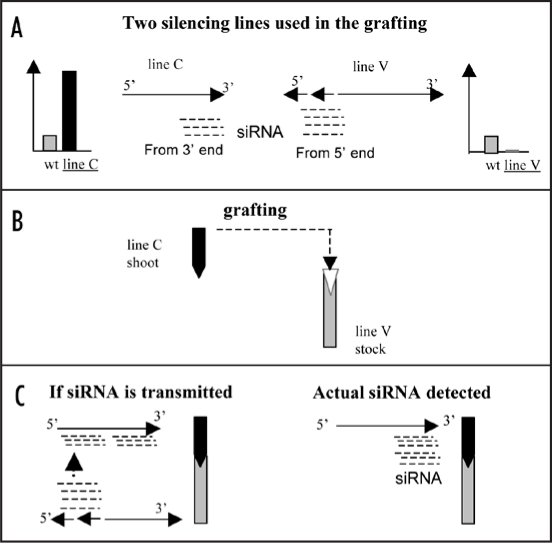
Figure 1.
Schematic diagram of grafting transmitted gene silencing of ACO1 in strong overexpresser line C after grafting to ACO1 strong silencer line V. (A) The ACO1 strong overexpresser line C contained a sense ACO1 transgene and showed much higher level of ACO1 mRNA (schematic) (light bar, wild type or wt; dark bar, line C) and also contained siRNAs from the 3′ region of the transgene (on the left).5 In contrast, the ACO1 strong silencer line V contained an ACO1 transgene with two inverted repeats (IRs) of the 5′ UTR region and siRNAs were mainly from the 5′ region of the transgene (on the right).5 (B) The shoot of the line C was grafted to line V stock. (C) SiRNAs corresponding to the 5′ region of ACO1 would be expected to be detected in the scion if the siRNAs from the stock line V were transmitted to the scion (on the left). However, grafting enhanced the generation of siRNAs from the 3′ region.4
More Efficient Silencing Induced by Grafting onto Strong Silencers and Self-Grafting
Since an ACO1 antisense (AS) line has similar features to the ACO1 overexpressers, i.e., a high level of the transgene AS mRNAs and accumulation of siRNAs, we investigated if grafting of the AS line to the ACO1 strong silencers would lead to further silencing of the AS transgene. For this AS transgene, it is clear that its mRNA undergoes degradation by posttranscriptional gene silencing (PTGS) as the endogenous ACO1 mRNA is silenced.5,7 The anti-sense ACO1 transgene produces two transcripts and the shorter one is due to an early alternative polyadenylation.7 This AS line accumulates much less siRNAs comparing to the strong sense silencer transgene, which is negatively correlated with the high level of transgene AS mRNA.5 This could indicate that the transgene mRNA may not be sensed as abRNA (it is designated to be capped and polyadenylated),7 otherwise, we would expect much more siRNA. In other words, the low level of siRNA may reflect a low level of abRNA in the AS line. Interestingly, the AS transgene mRNA is reduced after grafting (Fig. 2A), demonstrating that further silencing can be induced by grafting onto a silencing stock.
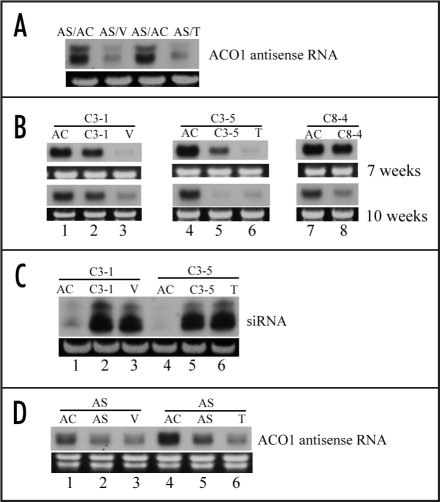
Figure 2.
Post-transcriptional gene silencing was enhanced in scions by grafting to a strong silencing stock and self-grafting. (A) The transmission of strong silencing from sense ACO1 silencing stocks to antisense ACO1 silencing scions. ACO1 antisense line (AS) shoots were grafted onto ACO1 strong silencer V or T lines as AS/V or AS/T, with AS shoots grafted onto wild type (Ailsa Craig) as controls (AS/AC).5,17 Total RNAs were extracted from the scions two weeks after grafting and analyzed using Northern hybridization for AS transgene mRNA level. The AS transgene generates two AS transcripts due to an alternative polyadenylation site.7 The levels of the two AS mRNAs were reduced in the scions (AS/V, AS/T) grafted to V or T lines, in contrast to those grafted to the wild type AC (AS/AC). Additional AS/V or AS/T graftings were carried out and showed similar results (data not shown). (B) Self-grafting of ACO1 overexpressers led to decreased level of ACO1 mRNA in scions. The ACO1 overexpressing lines C3-1, C3-5 and C8-4 containing siRNAs were self-grafted by cutting the shoots and grafting back to the same source plants using the same method as for heterografting, C3-1/C3-1 (lane 2), C3-5/C3-5 (lane 5) and C8-4/C8-4 (lane 8). ACO1 overexpressers C3-1, C3-5 and C8-4 were also grafted to wild type (AC) stock as negative controls (C3-1/AC, lane 1; C3-5/AC, lane 4; C8-4/AC, lane 7) and onto ACO1 strong silencer V or T lines as positive controls (C3-1/V, lane 3; C3-5/T, lane 6).4,5 Samples were taken for total RNA extraction and Northern analysis at seven weeks and 10 weeks after grafting. Self-grafted scions (C3-1/C3-1, lane 2; C3-5/C3-5, lane 5; C8-4/C8-4, lane 8) showed further silencing compared to grafting to wild type, especially by week 10. (C) siRNA accumulation in self-grafted scions (C3-1/C3-1, lane 2; C3-5/C3-5, lane 5) and scions grafted to strong silencers (C3-1/V, lane 3; C3-5/T, lane 6) increased compared to the scions grafted onto wild type stocks (C3-1/AC, lane 1; C3-5/C3-5, lane 4). Predominant small RNA stained with ethidium bromide is shown as equal loading controls. (D) ACO1 AS plants were self-grafted as in (B). Reduction in the AS mRNA was observed with AS self-grafting (AS/AS, lane 2 and 5) as for those grafted onto V or T lines (AS/V, lane 3; AS/T, lane 6), but not when grafted to wild type (AC) (lane 1, lane 4). Ethidium bromide staining of rRNA is shown as a control for equal loading for all the Northern hybridizations.
To test the nature of the silencing signal between the scion and stock in grafting transmitted silencing, we investigated if self-grafting would lead to enhanced silencing. We used both the ACO1 strong overexpressers, which produce siRNA, and the AS line, which has low levels of siRNAs.5 Comparing the expression level of the transgenes between scions grafted onto wild type and self-grafted scions, enhanced silencing by self-grafting was observed with the ACO1 overexpressers and the AS line (Fig. 2B and D). The enhanced silencing in ACO1 overexpressers by self-grafting was associated with dramatic increase of siRNAs (Fig. 2C).
Changes of Silencing States by Development
The further silencing induced by grafting as described above may be considered as a switch of silencing from one state to another. A change in the level of other components in the silencing pathway may also lead to the switching of silencing state. Low temperature may inactivate Dicer (DCL1) and inhibit siRNA generation, switching a silencing state to an inefficient silencing state.8 Silencing may also be influenced by development. Several cases have been reported where silencing is switched on at certain stages of development.9,10 Gene silencing of tobacco chitinase occurred during seedling development and the silenced genes were reset to a high expressing state in developing seeds 8–11 days post pollination.10 In a transgenic tomato line containing a trunctated polygalacturonase (PG) gene driven by the CaMV 35S promoter, siRNAs accumulate in leaves and green fruits, in which the endogenous PG gene is not expressed. The transgene mRNA accumulates at relatively high level in leaves and immature green fruits (before reaching the final fruit size) even though siRNAs accumulate. However, the transgene mRNA level is reduced dramatically in mature green fruits (final size) when PG gene is switched on.11,12 Such an influence of the switch-on of the endogenous PG gene transcription on the change of silencing state is supported further by the observation that the PG transgene silencing state did not change in the tomato mutant ripening inhibitor (rin), in which PG is not expressed in fruits.13 This strongly supports the suggestion that a transient increase of target mRNA by the increased transcription in the PG transgene silencing system leads to further and more efficient silencing.
We showed in our previous paper and this current study that silencing in scions that had high levels of target mRNA and contained siRNAs before grafting could be enhanced by grafting to strong silencer stocks (Fig. 2A).4 In other cases of grafting-transmitted silencing reported previously it is difficult to exclude the possibility that siRNAs may be present in the scion sources before grafting, as found in our experiments. Thus, a scion source with high level of target mRNA before grafting may actually be in a low efficiency silencing state with little or no decrease in target mRNA level or very low level of siRNAs, similar to that observed with ACO1 strong overexpresser.4 In a recent paper by Mourrain et al. it was found that the target mRNA is not silenced in one line (461–7/8) but grafting transmitted silencing occurred when it was grafted onto a silencing line (461–8).14 It is possible that the 461–7/8 scion source may contain a very low concentration of siRNAs because one of the parents is the silencing line 461–8.14 Therefore, grafting transmitted silencing may just be a switch of silencing states from weak silencing to stronger silencing, similar to the changes in silencing that occur during development.
The systemic silencing signal involved in the grafting transmitted silencing could be abRNAs as we proposed previously.4 Any RNAs that are different from normal RNAs with regard to structure [cap-, poly(A)], transcription termination (premature or late termination) and RNA binding proteins (e.g., exon junction complex) may be detected as aberrant RNAs.15 Such abRNAs may be recognized by RNA-dependent RNA polymerase (RdRP) as templates for antisense RNA production. For example, the transgene mRNA of the strong silencer Line V can form local double-stranded RNA (dsRNA) at the 5′ end, and cleavage of the dsRNA by Dicer would generate decapped ACO1 mRNA, which is suggested to be the characteristic of the templates for RdRP.5,16 Introduction of these decapped mRNAs into the ACO1 overexpresser scions by grafting may then help to increase the abRNA level, subsequently giving rise to a dramatic increase of siRNAs and strong silencing as we observed with the graftings of ACO1 overexpressers and the ACO1 antisense line over the line V stock (Fig. 2A).4 However, the success in enhancing silencing by self-grafting experiments using ACO1 overexpressers and the ACO1 antisense line suggests that an altered or new silencing signal between the scion and stock may not be so critical. Rather, the accumulation and sudden release of silencing signal at the grafting junction before and after the reconnection of the phloem, respectively, may be important for signal amplification. Both wounding sites (scion and stock) at the grafting junction may be sensed as a sink and silencing signal molecules such as abRNAs may be mobilised to these cells and accumulate, and subsequently dsRNA may be generated over the normal level. The reconnection of the phloem transport system, when the grafting junction between the stock and scion is healed, may create a wave of mobile RNAs, resulting from the combination of the relatively high level of these RNAs at both side of the junction, which then moves towards the shoot (the sink), breaking the previous silencing equilibrium and shifting it to a stronger silencing state. In our grafting experiments, transmission of enhanced silencing occurred as early as two weeks after grafting. Sensitive experiments designed to detect long aberrant RNAs and siRNAs at the grafting junction site at early stages (3–10 days) post grafting may provide evidence to test our hypothesis. Whatever the nature of the transmitted signal, it is clear that transmission cannot be explained solely by the movement of siRNAs, since it is the scion siRNAs that increase, not those from the stock (Fig. 1). Our hypothesis may also explain other grafting transmitted silencing, in which target mRNA level is high but siRNAs are not detected in the non-silencing scion source. It could be that the concentration of signal RNAs held up by the grafting junction in the silencing stock is still sufficient to trigger silencing in the scion once the barrier at the grafting junction is removed and the phloem is reconnected.
We have also considered whether wounding itself caused by grafting could stimulate silencing. Wounding alone during grafting does not seem to cause the change of silencing state, however, since we and other groups showed that scions, which showed grafting transmitted silencing when grafted to silencing stocks, did not show grafting transmitted silencing when grafted to wild type stock or non-silenced stock.1,4,6 Failure in grafting transmitted silencing in such grafting controls (testing scions grafted to non-silenced stocks) also indicates that the build-up of silencing signals on both sides of the grafting junction is critical.
In conclusion, there is evidence that one silencing state can be changed into another (stronger) silencing state by grafting or development. We propose that the dramatic release of accumulated silencing signal RNAs at the grafting junction, when the phloem between the scion and stock is re-connected, may be the key to the grafting transmitted silencing.
Acknowledgements
The work was supported by BBSRC (BBS/B/10846).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/4814
References
- 1.Palauqui JC, Elmayan T, Pollien JM, Vaucheret H. Systemic acquired silencing: Transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 1997;15:4738–4745. doi: 10.1093/emboj/16.15.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García-Pérez RD, Van Houdt H, Depicker A. Spreading of posttranscriptional gene silencing along the target gene promotes systemic silencing. Plant J. 2004;38:594–602. doi: 10.1111/j.1365-313X.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 3.Tournier B, Tabler M, Kalantidis K. Phloem flow strongly influences the systemic spread of silencing in GFP Nicotiana benthamiana plants. Plant J. 2006;47:383–394. doi: 10.1111/j.1365-313X.2006.02796.x. [DOI] [PubMed] [Google Scholar]
- 4.Shaharuddin NA, Han Y, Li H, Grierson D. The mechanism of graft transmission of sense and antisense gene silencing in tomato plants. FEBS Lett. 2006;580:6579–6586. doi: 10.1016/j.febslet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Han Y, Grierson D. The influence of inverted repeats on the production of small antisense RNAs involved in gene silencing. Mol Genet and Genom. 2002;267:629–635. doi: 10.1007/s00438-002-0696-z. [DOI] [PubMed] [Google Scholar]
- 6.Sonoda S, Nishiguchi M. Graft transmission of post-transcriptional gene silencing: Target specificity for RNA degradation is transmissible between silenced and non-silenced plants, but not between silenced plants. Plant J. 2000;21:1–8. doi: 10.1046/j.1365-313x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton A, Lycett GW, Grierson D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature. 1990;346:284–287. [Google Scholar]
- 8.Szittya G, Silhavy D, Molnár A, Havelda Z, Lovas Á, Lakatos L, Bánfalvi Z, Burgyán J. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 2003;22:633–640. doi: 10.1093/emboj/cdg74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boerjan W, Bauw G, Van Montagu M, Inzé D. Distinct phenotypes generated by overexpression and suppression of S-adenosyl-L-methionine synthetase reveal developmental patterns of gene silencing in tobacco. Plant Cell. 1994;6:1401–1414. doi: 10.1105/tpc.6.10.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunz C, Schob H, Stam M, Kooter JM, Meins FJ. Developmentally regulated silencing and reactivation of tobacco chitinase transgene expression. Plant J. 1996;10:437–450. [Google Scholar]
- 11.Han Y, Grierson D. Relationship between small antisense RNAs and aberrant RNAs associated with sense transgene mediated gene silencing in tomato. Plant J. 2002;29:509–519. doi: 10.1046/j.1365-313x.2002.01236.x. [DOI] [PubMed] [Google Scholar]
- 12.Grierson D, Tucker GA. Timing of ethylene and polygalacturonase synthesis in relation to the control of tomato fruit ripening. Planta. 1983;157:174–179. doi: 10.1007/BF00393652. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Griffiths A, Li H, Grierson D. The effect of endogenous mRNA levels on co-suppression in tomato. FEBS Lett. 2004;563:123–128. doi: 10.1016/S0014-5793(04)00280-7. [DOI] [PubMed] [Google Scholar]
- 14.Mourrain P, van Blokland R, Kooter JM, Vaucheret H. A single transgene locus triggers both transcriptional and post-transcriptional silencing through double-stranded RNA production. Planta. 2007;225:365–379. doi: 10.1007/s00425-006-0366-1. [DOI] [PubMed] [Google Scholar]
- 15.Herr AJ, Molnár A, Jones A, Baulcombe DC. Defective RNA processing enhances RNA silencing and influences flowering of Arabidopsis. Proc Natl Acad Sci USA. 2006;103:14994–15001. doi: 10.1073/pnas.0606536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazzani S, Lawrenson T, Woodward C, Headon D, Sablowski R. A link between mRNA turnover and RNA interference in arabidopsis. Science. 2004;306:1046–1048. doi: 10.1126/science.1101092. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton AJ, Brown S, Han Y, Ishizuka M, Lowe A, Alpuche-solis AG, Grierson D. A transgene with repeated DNA causes high frequency, post-transcriptional suppression of ACC-oxidase gene expression in tomato. Plant J. 1998;15:737–746. doi: 10.1046/j.1365-313X.1998.00251.x. [DOI] [PubMed] [Google Scholar]