Abstract
Legume plants have a unique ability to fix atmospheric nitrogen via symbiosis with rhizobia. For the establishment of symbiosis, legume plants secrete signaling molecules such as flavonoids from root tissues, leading to the attraction of rhizobia and the induction of rhizobial nod genes. Genistein and daidzein are found in soybean root exudates and function as signal molecules in soybean-Bradyrhizobium japonicum chemical communication. Although it is more than 20 years since these signal flavonoids were identified, almost nothing has been characterized concerning the membrane transport process of these molecules from soybean roots. To elucidate the transport mechanism we performed membrane transport assays with plasma membrane-enriched vesicles and various inhibitors. As a result, we concluded that an ATP-binding cassette-type transporter is involved in the secretion of genistein from soybean roots. The possible involvement of a pleiotropic drug resistance-type ABC transporter in this secretion is also discussed.
Key words: symbiotic nitrogen fixation, soybean, genistein, root exudates, ABC transporter
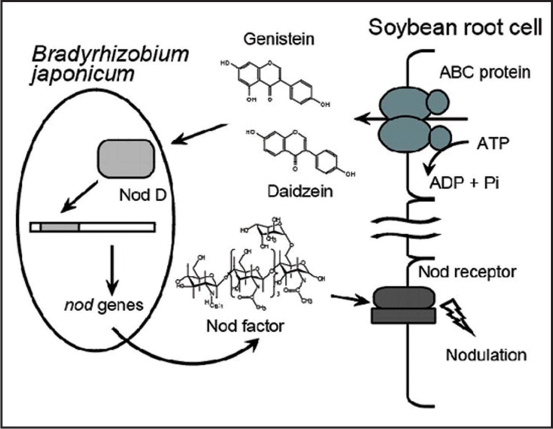
Legumes (Fabaceae) constitute the third largest plant family, behind only orchids (Orchidaceae) and asters (Asteraceae), with around 700 genera and 20,000 species.1 From the viewpoint of agricultural importance, they represent the largest plant family among dicots, whereas Gramineae is the counterpart in monocots that contain most crop plants. A hallmark feature of legume plants is their ability to gain nitrogen nutrients directly from soil microbes called rhizobia through symbiosis. The plant-rhizobium symbiosis takes place in specialized organs, nodules, where rhizobia convert atmospheric nitrogen effectively into ammonium to facilitate nitrogen fixation in amino acids, which is beneficial for the host plants. In return the plants supply the rhizobia with the photosynthetic products, carbohydrates.2 The inter-recognition via chemical signals between a host plant and rhizobium is highly specific for both species and is indispensable for the development of functional nodules. These sophisticated signaling cascades begin with the secretion of flavonoids from the roots of the host plant,3–5 which are recognized by the NodD protein in the rhizobia leading to the successive induction of nod gene expression to produce the second signaling molecules, lipochitooligosaccharides, also known as Nod-factors (Fig. 1). Nod-factors perceived by receptors in the host plant induce various signaling events such as ‘calcium spiking’ response, ultimately resulting in the formation of nodules.6
Figure 1.
A model of flavonoid secretion from soybean roots and the inter-recognition between legume and rhizobium.
As signaling flavonoids, genistein and daidzein were identified in root exudates of soybean many years ago,7,8 and indeed an appreciable amount of these flavonoid aglycons as well as their glucosides were detected by LC-MS analysis in the hydroponic culture media in our study. However, almost nothing is known about the membrane transport involved in flavonoid secretion from legume roots. To elucidate the transport mechanism of flavonoid secretion from the root tissues of legume plants, we used soybean and genistein as a model system and performed biochemical analyses using root-membrane-vesicle transport experiments. Using a plasma membrane-enriched fraction, we detected clear ATP-dependent transport activity of genistein, and demonstrated with various transport inhibitors that an ATP-binding cassette (ABC)-type transporter mediates the secretion of flavonoids from soybean roots (Fig. 1).9
ABC transporters constitute the largest family of transporters in plants, with more than 120 members in Arabidopsis and rice, and at least 91 members in a model legume, Lotus japonicus.10–12 ABC transporters are defined by a highly conserved domain called the nucleotide binding domain (NBD) which consists of a Walker A box [GX4GK(ST)], a Walker B box [(RK)X3GX3L (hydrophobic)3],13 and an ABC signature [(LIVMFY)S(SG)GX3(RKA)(LIVMYA)X(LIVFM)(AG)] between them.14 Most ABC transporters are membrane proteins with one or two transmembrane domains (TMDs), while ‘soluble’ ABC transporters without TMDs are also found. Contrary to the general understanding that ABC transporters are multispecific transporters, no typical multiple drug efflux pump has been reported among plant ABC transporters, but they seem to play specific and diverse roles in plant life. For instance, stomata movement,15,16 wax secretion,17 alkaloid translocation,18 and iron homeostasis19,20 are all reported as functions of specific ABC transporters in plants. ABC members involved in the transport of signal molecules are also known, e.g., Ste6 for a yeast mating hormone,21 and AtPGP1, AtPGP4, and AtPGP19 for auxin transport.22–24 As a large number of ABC transporters remain still to be characterized, direct relevance to signal-compound secretion in plants will be further uncovered in the future.
Although there are some potent inhibitors of ABCB subfamily members and a general enhancing element, glutathione, is also known for the ABCC subfamily, our transport assays suggested that the ABC transporter of a non-ABCB or non-ABCC-type member was involved in genistein secretion.9 It could be speculated that another subfamily, e.g., pleiotropic drug resistance (PDR)-type ABC transporters, which are ‘full-size’ proteins with an NBD1-TMD1-NBD2-TMD2 structure, may be involved in genistein secretion. In fact, a higher number of PDR subfamily members than any other subfamily are expressed in soybean roots. PDR-type ABC transporters are specific to plants and yeast but are not found in animals or bacteria, although ‘half-size’ ABCG members with NBD-TMD orientation share sequence similarities with PDR-type ABCs and are found in human.25 Human ABCG members are well studied due to their clinical importance in lipid homeostasis26 and several inhibitors, such as novobiocin and estrone, have been reported for the ABCG2 protein.27,28 We have tested whether these chemicals could inhibit genistein transport, but they failed to inhibit the transport activity. It is probable that these inhibitors are specific to human ABCG members, especially to ABCG2, and may not inhibit the activity of plant PDR members. Development of specific inhibitors for plant PDR members is required to conclude the direct involvement of PDR members in the secretion of flavonoids, but an informatics approach with genomic and expressed sequence tag (EST) information can be applied. In the soybean gene index (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=soybean), we found 13 PDR genes expressed in roots: TC204797, TC204798, TC204799, TC208020, TC210090, TC211803, TC218050, TC218747, TC218748, TC222885, TC227242, TC227243, TC231151 and AW831708. Functional analysis of these genes with a heterologous expression system and/or physiological studies on transgenic soybean plants with RNAi knock down technology may enable us to identify the gene(s) responsible for genistein secretion.
Legume plants are unique in their ability to assimilate atmospheric nitrogen through symbiosis with rhizobia, and are important to our lives. Research on legume plants is becoming very active; more information on genome sequences, genetic markers and expression data from cDNA arrays have been accumulated worldwide, which led to the identification of important proteins functioning in the signal recognition and transduction for the establishment of nodules.29,30 This information will also be used to identify the protein responsible for the secretion of signal flavonoids as well as phytoalexins in the near future.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/4819
References
- 1.Doyle JJ, Luckow MA. The rest of the iceberg: Legume diversity and evolution in a phylogenetic context. Plant Physiol. 2003;131:900–910. doi: 10.1104/pp.102.018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prell J, Poole P. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 2006;14:161–168. doi: 10.1016/j.tim.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Firmin JL, Wilson KE, Rossen L, Johnston AWB. Flavonoid activation of nodulation genes in Rhizobium reversed by other compounds present in plants. Nature. 1986;324:90–92. [Google Scholar]
- 4.Peters NK, Frost JW, Long SR. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986;233:977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- 5.Redmond J, Batley M, Djordjevic M, Innes R, Kuempel P, Rolfe B. Flavones induce expression of nodulation genes in Rhizobium. Nature. 1986;323:632–635. [Google Scholar]
- 6.Geurts R, Fedorova E, Bisseling T. Nod factor signaling genes and their function in the early stages of Rhizobium infection. Curr Opin Plant Biol. 2005;8:346–352. doi: 10.1016/j.pbi.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Kosslak RM, Bookland R, Barkei J, Paaren HE, Appelbaum ER. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci USA. 1987;84:7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smit G, Puvanesarajah V, Carlson RW, Barbour WM, Stacey G. Bradyrhizobium japonicum nodD1 can be specifically induced by soybean flavonoids that do not induce the nodYABC-SUIJ operon. J Biol Chem. 1992;267:310–318. [PubMed] [Google Scholar]
- 9.Sugiyama A, Shitan N, Yazaki K. Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in Legume-Rhizobium symbiosis. Plant Physiol. 2007;144:2000–2008. doi: 10.1104/pp.107.096727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Fernandez R, Davies TG, Coleman JO, Rea PA. The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J Biol Chem. 2001;276:30231–30244. doi: 10.1074/jbc.M103104200. [DOI] [PubMed] [Google Scholar]
- 11.Garcia O, Bouige P, Forestier C, Dassa E. Inventory and comparative analysis of rice and Arabidopsis ATP-binding cassette (ABC) systems. J Mol Biol. 2004;343:249–265. doi: 10.1016/j.jmb.2004.07.093. [DOI] [PubMed] [Google Scholar]
- 12.Sugiyama A, Shitan N, Sato S, Nakamura Y, Tabata S, Yazaki K. Genome-wide analysis of ATP-binding cassette (ABC) proteins in a model legume plant, Lotus japonicus: Comparison with Arabidopsis ABC protein family. DNA Res. 2006;13:205–228. doi: 10.1093/dnares/dsl013. [DOI] [PubMed] [Google Scholar]
- 13.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bairoch A. PROSITE: A dictionary of sites and patterns in proteins. Nucleic Acids Res. 1992;20:2013–2018. doi: 10.1093/nar/20.suppl.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaedeke N, Klein M, Kolukisaoglu U, Forestier C, Muller A, Ansorge M, Becker D, Mamnun Y, Kuchler K, Schulz B, Mueller-Roeber B, Martinoia E. The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J. 2001;20:1875–1887. doi: 10.1093/emboj/20.8.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein M, Geisler M, Suh SJ, Kolukisaoglu HU, Azevedo L, Plaza S, Curtis MD, Richter A, Weder B, Schulz B, Martinoia E. Disruption of AtMRP4, a guard cell plasma membrane ABCC-type ABC transporter, leads to deregulation of stomatal opening and increased drought susceptibility. Plant J. 2004;39:219–236. doi: 10.1111/j.1365-313X.2004.02125.x. [DOI] [PubMed] [Google Scholar]
- 17.Pighin JA, Zheng H, Balakshin LJ, Goodman IP, Western TL, Jetter R, Kunst L, Samuels AL. Plant cuticular lipid export requires an ABC transporter. Science. 2004;306:702–704. doi: 10.1126/science.1102331. [DOI] [PubMed] [Google Scholar]
- 18.Shitan N, Bazin I, Dan K, Obata K, Kigawa K, Ueda K, Sato F, Forestier C, Yazaki K. Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica. Proc Natl Acad Sci USA. 2003;100:751–756. doi: 10.1073/pnas.0134257100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi H, Nishizawa NK, Nakanishi H, Mori S. IDI7, a new iron-regulated ABC transporter from barley roots, localizes to the tonoplast. J Exp Bot. 2002;53:727–735. doi: 10.1093/jexbot/53.369.727. [DOI] [PubMed] [Google Scholar]
- 20.Ducos E, Fraysse S, Boutry M. NtPDR3, an iron-deficiency inducible ABC transporter in Nicotiana tabacum. FEBS Lett. 2005;579:6791–6795. doi: 10.1016/j.febslet.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Kuchler K, Sterne RE, Thorner J. Saccharomyces cerevisiae STE6 gene product: A novel pathway for protein export in eukaryotic cells. EMBO J. 1989;8:3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terasaka K, Blakeslee JJ, Titapiwatanakun B, Peer WA, Bandyopadhyay A, Makam SN, Lee OR, Richards EL, Murphy AS, Sato F, Yazaki K. PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell. 2005;17:2922–2939. doi: 10.1105/tpc.105.035816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, Ejendal KF, Smith AP, Baroux C, Grossniklaus U, Muller A, Hrycyna CA, Dudler R, Murphy AS, Martinoia E. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005;44:179–194. doi: 10.1111/j.1365-313X.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- 24.Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J, Geisler M, Nagashima A, Sakai T, Martinoia E, Friml J, Peer WA, Murphy AS. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell. 2007;19:131–147. doi: 10.1105/tpc.106.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Peng H, Zhang JT. Human multidrug transporter ABCG2, a target for sensitizing drug resistance in cancer chemotherapy. Curr Med Chem. 2007;14:689–701. doi: 10.2174/092986707780059580. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto Y, Tsukahara S, Imai Y, Sugimoto Y, Ueda K, Tsuruo T. Reversal of breast cancer resistance protein-mediated drug resistance by estrogen antagonists and agonists. Mol Cancer Ther. 2003;2:105–112. [PubMed] [Google Scholar]
- 28.Shiozawa K, Oka M, Soda H, Yoshikawa M, Ikegami Y, Tsurutani J, Nakatomi K, Nakamura Y, Doi S, Kitazaki T, Mizuta Y, Murase K, Yoshida H, Ross DD, Kohno S. Reversal of breast cancer resistance protein (BCRP/ABCG2)-mediated drug resistance by novobiocin, a coumermycin antibiotic. Int J Cancer. 2004;108:146–151. doi: 10.1002/ijc.11528. [DOI] [PubMed] [Google Scholar]
- 29.Stacey G, Libault M, Brechenmacher L, Wan J, May GD. Genetics and functional genomics of legume nodulation. Curr Opin Plant Biol. 2006;9:110–121. doi: 10.1016/j.pbi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Sato S, Nakamura Y, Asamizu E, Isobe S, Tabata S. Genome sequencing and genome resources in model legumes. Plant Physiol. 2007;144:588–593. doi: 10.1104/pp.107.097493. [DOI] [PMC free article] [PubMed] [Google Scholar]