Abstract
ROPs/RACs are the only known signaling Ras superfamily small GTPases in plants. As such they have been suggested to function as central regulators of diverse signaling cascades. The ROP/RAC signaling networks are largely unknown, however, because only few of their effector proteins have been identified. In a paper that was published in the June 5, 2007 issue of Current Biology we described the identification of a novel ROP/RAC effector designated ICR1 (Interactor of Constitutive active ROPs 1). We demonstrated that ICR1 functions as a scaffold that interacts with diverse but specific group of proteins including SEC3 subunit of the exocyst vesicle tethering complex. ICR1-SEC3 complexes can interact with ROPs in vivo and are thereby recruited to the plasma membrane. ICR1 knockdown or silencing leads to cell deformation and loss of the root stem cells population, and ectopic expression of ICR1 phenocopies activated ROPs/RACs. ICR1 presents a new paradigm in ROP/RAC signaling and integrates mechanisms regulating cell form and pattern formation at the whole plant level.
Key words: Rho, auxin, root development, vesicle trafficking, RAC, ROP, polarity, Arabidopsis, exocyst
Introduction
Cellular polarity governs cell growth and shape, the orientation of cell division, cell differentiation and pattern formation of multi-cellular organisms. The Rho family of small GTPases have emerged as master regulators of cell polarity that coordinate actin organization and dynamics, vesicle trafficking as well as establishment of Ca2+ and ROS gradients.1 Plants posses a single Rho subfamily, designated either ROPs (Rho of Plants) or RACs.2,3 Studies using dominant active mutants ROP/RAC demonstrated that these proteins regulate cell shape by enhancement of actin polymerization, inhibition of micro-tubule polymerization, induction of Ca2+ gradients and inhibition vesicle cycling at the plasma membrane.4–11 ROPs/RACs have been shown to localize at the apex of tip growing cells and at the cell plate in dividing cells.6,12 Some activated ROPs/RACs are transiently S-acylated and consequently partition into detergent resistant membranes (DRM) that are likely to be lipid rafts.13 Lipid rafts are membrane microdomains that act as platforms for fast exocytosis and regulate membrane trafficking.14,15 Thus, ROP/RACs are suggested to direct the exocytotic machinery and the cytoskeleton to the sites of fast membrane expansion.
In yeast, Rho1, Rho3 and Cdc42 regulate late exocytosis events by interaction with subunits of the exocyst vesicle tethering complex.16–19 All the putative subunits of the exocyst complex have been identified in plants but direct interaction of ROP/RACs with either exocyst subunits has not been reported.20,21 Auxin dependent signaling was shown to determine localization of ROP/RACs in trichoblast prior to root hair initiation.22 A direct role for ROPs/RACs in pattern formation has not been established, however, likely due to their functional redundancy and the fact that only limited number of their effectors have been identified.
Results and Discussion
In a paper that was recently published in Current Biology23 we described the identification of a novel ROP/RAC effector that was designated ICR1 (Interactor of Constitutive active ROP 1). ICR1 is a member of a small family consisting of five proteins that are composed of coiled coil domains and no other recognizable catalytic or structural domains (Lavy and Yalovsky, unpublished data). The coiled coil domains of the ICRs bear similarity to the coiled coil domain of Rho associated Coiled Coil containing Kinase 1 (ROCK1),24 suggesting that it is an ancient interaction module of Rho GTPases. ICR1 interacted with the GTP-bound activated ROPs/RACs in vitro, in yeast and in plants. Bimolecular Fluorescence Complementation (BiFC) and colocalization assays demonstrated that ROPs/RACs recruit ICR1 to the plasma membrane.23 Coiled coil proteins have emerged as key regulators of vesicle trafficking acting as scaffolds for the formation of multi-protein complexes. We therefore hypothesized that ICR1 could be an important link between the ROP/RAC GTPases and specific proteins or protein complexes.
To test our hypothesis we searched for proteins, which interact with ICR1 and ICR2, using either one as bait in yeast two-hybrid screens. Either of the two ICRs interacted with completely different set of proteins (Lavy and Yalovsky, unpublished data). Furthermore, ICR1 interacted with itself but not with ICR2. ICR2, however, did not interact with itself.23 These results suggested that ICR1 and ICR2 have unique functions. The data further suggested that the ICRs function as scaffolds, responsible for the formation of specific protein complexes that are recruited to the plasma membrane by ROPs.
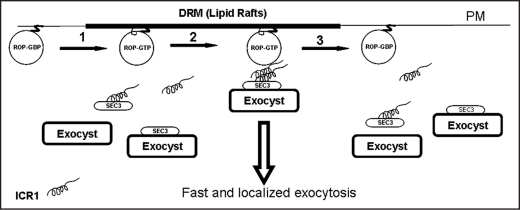
Two of the proteins that were identified in the screens with ICR1 as bait were AtSEC3A subunit of the exocyst23 complex and reassuringly AtROP2 (Lavy and Yalovsky, unpublished data). We further demonstrated that when transiently expressed in Nicotiana bethamiana leaf epidermal cells, ICR1 and AtSEC3A form complexes in the cytoplasm and that following coexpression with AtROP9 the ICR1-AtSEC3A complexes are recruited to the plasma membrane.23 These results strongly suggested that ICR1 forms the missing link between ROP/RAC GTPases and the exocyst complex in plants (Fig. 1).
Figure 1.
Possible function of ICR1 and ROPs in localized exocytosis. (1) Activated, GTP-bound, ROPs are transiently acylated on an internal cysteine residue and as a result partition into detergent resistant membranes (DRM) that could be lipid rafts.13 (2) ICR1 monomer or dimer interacts with SEC3 in the cytoplasm and in turn this complex interacts with ROPs at the plasma membrane.23 The ROP-ICR1-SEC3 complex could then serve a landmark for the formation of the exocyst complex. Alternatively, ICR1 may interact with SEC3-exocyst complex or may be a part of the exocyst complex or sub-complex. (3) Upon GTP hydrolysis the ICR1 is released from the ROP and the ICR1-SEC3-exocyst complexes return back to the cytoplasm. The ROP is deacylated and partitions into detergent soluble membranes. The rate of ROP activation/inactivation cycle depends primarily on ROP-GAPs29 and ROP-GEFs and the possible activation/inactivation of the latter by receptor protein kinases in the plasma membrane.30,31 In addition to prenylation and transient acylation, membrane localization of ROPs very likely depends on function of RhoGDIs32 and interaction of the C-terminal polybasic domains of ROPs with phosphatidylphosphoinositides (PIPs).33
ICR1 is predicted to contain two coiled coil domains. A mutant in the C-terminal domain compromised dimerization of ICR1 and its interaction with ROPs and AtSEC3A.23 Ectopic expression of ICR1 in Arabidopsis induced cell and organ deformation very similar to those that are induced by activated ROP/RAC mutants. Trasngenic plants expressing the C-terminal coiled-coil domain icr1 mutant were virtually indistinguishable from wild type plants, indicating that the capability to interact with ROPs/RACs and SEC3 is required for ICR1 gain of function.23 Mutations that disrupted the N-terminal coiled-coil domain of ICR1 compromised its homodimrization but not its interaction with either ROPs or AtSEC3A.23 This raises an interesting possibility that dimerization of ICR1 may not be required for its interaction with ROPs and AtSEC3, implying that an ICR1 monomer could be part of the exocyst complex (Fig. 1).
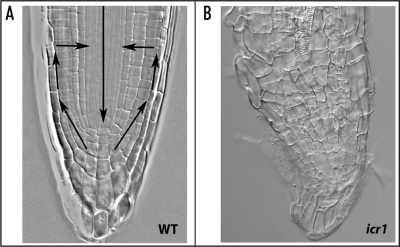
ICR1 loss of function in a T-DNA insertion mutant and RNAi plants leads to growth arrest of the primary root and development of numerous adventitious roots.23 Similar to mutants with compromised polar auxin transport,25,26 the root cap cells of icr1 mutant and RNAi plants loose the columella identity and root hairs develop close to the apical root tip. The adventitious roots develop to length of few centimeters and then, as in the primary roots, their apical meristem collapses and their growth ceases. Thus, the collapse of the root apical meristem in icr1 mutant and RNAi plants is a gradual process, suggesting that polar auxin transport could be compromised (Fig. 2).27,28 The phenotypes of the ICR1 mutant and RNAi plants and of the ICR1 overexpressing plants indicate that ROPs/RAC GTPases regulate molecular mechanisms that affect both cell structure as well as pattern formation at the whole plant level.
Figure 2.
ICR1 maybe part of a polar auxin transport mechanism. The maintenance of the root apical meristem depends on polar auxin transport and formation of an auxin maximum at the root tip27,28 (arrows, A). The collapse of the root apical meristem in icr1 mutant seedlings (B) suggests that ICR1 is required for polar auxin transport.
The ICRs expands the plethora of ROP/RAC effector proteins. Their function as scaffolds that interact with a wide array yet specific groups of proteins, present a new paradigm of ROP/RAC function. Our future studies will be aimed at identifying the protein complexes in which the ICRs function, how the interactions of ICRs with different proteins are regulated and define the processes that are regulated by the ROP/RAC and ICRs.
Acknowledgements
The research described in this paper was supported by the Charles Revson fund of the Israel Science Foundation (ISF-399/03) and The German Israeli DIP program grants to S.Y. M.L. was a recipient of the Israel Ministry of Science Eshkol and The Manna Center for Plant Biosciences, Tel Aviv University fellowships.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/4838
References
- 1.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: Regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen TM, Vejlupkova Z, Sharma YK, Arthur KM, Spatafora JW, Albright CA, Meeley RB, Duvick JP, Quatrano RS, Fowler JE. Conserved subgroups and developmental regulation in the monocot rop gene family. Plant Physiol. 2003;133:1791–1808. doi: 10.1104/pp.103.029900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winge P, Brembu T, Kristensen R, Bones AM. Genetic structure and evolution of RAC-GTPases in Arabidopsis thaliana. Genetics. 2000;156:1959–1971. doi: 10.1093/genetics/156.4.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Lin Y, Heath RM, Zhu MX, Yang Z. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell. 1999;11:1731–1742. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Y, Wu G, Yang Z. Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J Cell Biol. 2001;152:1019–1032. doi: 10.1083/jcb.152.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molendijk AJ, Bischoff F, Rajendrakumar CS, Friml J, Braun M, Gilroy S, Palme K. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 2001;20:2779–2788. doi: 10.1093/emboj/20.11.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y, Li H, Yang Z. The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell. 2002;14:777–794. doi: 10.1105/tpc.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones MA, Shen J-J, Fu Y, Li H, Yang Z, Grierson CS. The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell. 2002;14:763–776. doi: 10.1105/tpc.010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol. 2005;169:127–138. doi: 10.1083/jcb.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Bloch D, Lavy M, Efrat Y, Efroni I, Bracha-Drori K, Abu-Abied M, Sadot E, Yalovsky S. Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling. Mol Biol Cell. 2005;16:1913–1927. doi: 10.1091/mbc.E04-07-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y, Wang Y, Zhu J-K, Yang Z. Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell. 1996;8:293–303. doi: 10.1105/tpc.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorek N, Poraty L, Sternberg H, Bar E, Lewinsohn E, Yalovsky S. Activation status-coupled transient S acylation determines membrane partitioning of a plant Rho-related GTPase. Mol Cell Biol. 2007;27:2144–2154. doi: 10.1128/MCB.02347-06. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581:2098–2104. doi: 10.1016/j.febslet.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 16.TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 17.Guo W, Tamanoi F, Novick P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol. 2001;3:353–360. doi: 10.1038/35070029. [DOI] [PubMed] [Google Scholar]
- 18.Wiederkehr A, Du Y, Pypaert M, Ferro-Novick S, Novick P. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol Biol Cell. 2003;14:4770–4782. doi: 10.1091/mbc.E03-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novick P, Guo W. Ras family therapy: Rab, Rho and Ral talk to the exocyst. Trends Cell Biol. 2002;12:247–249. doi: 10.1016/s0962-8924(02)02293-6. [DOI] [PubMed] [Google Scholar]
- 20.Elias M, Drdova E, Ziak D, Bavlnka B, Hala M, Cvrckova F, Soukupova H, Zarsky V. The exocyst complex in plants. Cell Biol Int. 2003;27:199–201. doi: 10.1016/s1065-6995(02)00349-9. [DOI] [PubMed] [Google Scholar]
- 21.Cole RA, Synek L, Zarsky V, Fowler JE. SEC8, a subunit of the putative Arabidopsis exocyst complex, facilitates pollen germination and competitive pollen tube growth. Plant Physiol. 2005;138:2005–2018. doi: 10.1104/pp.105.062273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer U, Ikeda Y, Ljung K, Serralbo O, Singh M, Heidstra R, Palme K, Scheres B, Grebe M. Vectorial information for Arabidopsis planar polarity is mediated by combined AUX1, EIN2, and GNOM activity. Curr Biol. 2006;16:2143–2149. doi: 10.1016/j.cub.2006.08.091. [DOI] [PubMed] [Google Scholar]
- 23.Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S. A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol. 2007;17:947–952. doi: 10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 24.Dvorsky R, Blumenstein L, Vetter IR, Ahmadian MR. Structural insights into the interaction of ROCKI with the switch regions of RhoA. J Biol Chem. 2004;279:7098–7104. doi: 10.1074/jbc.M311911200. [DOI] [PubMed] [Google Scholar]
- 25.Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development. 2001;128:4057–4067. doi: 10.1242/dev.128.20.4057. [DOI] [PubMed] [Google Scholar]
- 26.Benjamins R, Ampudia CS, Hooykaas PJ, Offringa R. PINOID-mediated signaling involves calcium-binding proteins. Plant Physiol. 2003;132:1623–1630. doi: 10.1104/pp.103.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 28.Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell. 2006;18:3033–3046. doi: 10.1105/tpc.106.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaothien P, Ok SH, Shuai B, Wengier D, Cotter R, Kelley D, Kiriakopolos S, Muschietti J, McCormick S. Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J. 2005;42:492–503. doi: 10.1111/j.1365-313X.2005.02388.x. [DOI] [PubMed] [Google Scholar]
- 31.Shichrur K, Yalovsky S. Turning ON the switch-RhoGEFs in plants. Trends Plant Sci. 2006;11:57–59. doi: 10.1016/j.tplants.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Klahre U, Becker C, Schmitt AC, Kost B. Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. Plant J. 2006;46:1018–1031. doi: 10.1111/j.1365-313X.2006.02757.x. [DOI] [PubMed] [Google Scholar]
- 33.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]