Abstract
We have recently reported that ozone (O3) can inhibit mitochondrial respiration and induce activation of the alternative oxidase (AOX) pathway and in particular AOX1a in tobacco. While O3 causes mitochondrial H2O2, early leaf nitric oxide (NO) as well as transient ethylene (ET) accumulation, the levels of jasmonic acid and 12-oxo-phytodienoic acid remained unchanged. It was shown that both, NO and ET dependent pathways can induce AOX1a transcription by O3. AOX plays a role in reducing reactive oxygen species (ROS) which in turn are linked to biotic and abiotic plant stresses, much like the second messengers guanosine 3′, 5′-cyclic monophosphate (cGMP). The goal is to unravel specific cGMP signatures and induction pathways downstream from O3 and NO, including transcription of AOX1a. Here we propose that some late (>3 h) responses to NO, e.g., the accumulation of phenylalanine lyase (PAL) transcripts, are critically cGMP dependent, while the early (<2 h) responses, including AOX1a induction are not.
Key words: ozone, nitric oxide, ethylene, alternative oxidase (AOX), cGMP, Nicotiana tabacum L., Arabidopsis thaliana
A Role for Aox in Plant Stress Responses
It is conceivable that a function of the AOX pathway is to reduce the formation of ROS whereby increased activity of AOX could relieve the mitochondrial cytochrome pathway and prevent harmful hyper-reduction, reducing the formation of damaging radicals.1,2 ROS generation is also involved in biotic and abiotic stresses responses in plants. While AOX abundance and AOX activity are low in unstressed plants, alternative respiration is enhanced after various developmental or environmental stimuli, e.g., under conditions such as wounding and plant disease1 thus implicating AOX in stress alleviation. The nuclear gene that encodes AOX in tobacco (Nicotiana tabacum; AOX1) is rapidly induced in cultured cells when the cytochrome pathway is specifically inhibited by antimycin A.3
Addition of salicylic acid (SA) to tobacco cell suspensions or intact leaves also induces gene the expression of AOX associated genes4 further linking AOX to plant stress responses. More recently NO has been identified as a key molecule that interacts with ROS in a number of ways, either as a crucial partner in determining cell fate or in signaling in response to developmental and stress-related conditions. NO appears to be involved in controlling or modulating various aspects of plant pathogen resistance, growth, development, and senescence, as well as stomatal movement.5
In NO treated Arabidopsis cell cultures expression is strongly induced, resulting in increased respiration through the alternative pathway.6 Furthermore, AOX1a expression is affected in the Arabidopsis ctr-1 mutant thus pointing to ethylene (ET) dependence.7
Different signaling molecules have been found to be involved in AOX induction, but their interactions during environmental stresses remain unresolved. Analyses of Arabidopsis mutants have produced a substantial body of information implicating SA, NO, ROS, jasmonic acid (JA), and ET as major endogenous signals in defense responses. However, much less information is available for other plant species. Our previous studies demonstrated that O3 treatment induces SA and H2O2 accumulation in the O3-sensitive tobacco BelW3 cultivar,8 and the activation of a cell death program9 as well as the induction of the AOX pathway by O3 in tobacco.10,11
Early (1–1.5 h) production of NO in fumigated leaves was detected with a NO-specific fluorescence concomitant with H2O2 accumulation in mitochondria and ET evolution was recorded during O3 exposure. In summary, while it is established that NO is the main upstream signaling molecule involved in AOX1a expression, which is coordinately activated by ET, the exact nature of the downstream signaling pathway remains to be elucidated.
Linking cGMP to Stress Responses
It is becoming increasingly clear that the cyclic nucleotide cGMP has an important role in many diverse biological processes12–14 including responses to both abiotic and biotic stresses,15,16 and NO dependent signaling5,17,18 as well as the regulation of transcription.19
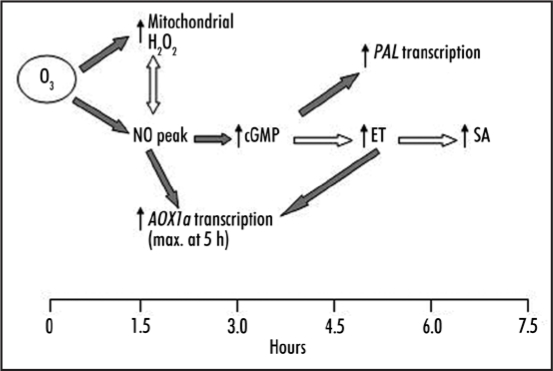
It is interesting to note, that the time for cGMP increases does vary considerably from 15 min in response to NaCl and osmotica15 to 2 h after application of an NO donor,16 and 5 h in response to a gravitropic stimulus.20 This can be taken as an indication for the complex and differentiated role of cGMP as signaling molecule. Our current experiments suggest that in Tobacco significant rises in cGMP levels occur only >3 h after NO fumigation. This would suggest that cGMP has no role in NO dependent AOX1a induction. Inspecting microarray data in the public domain we noted that in Arabidopsis thaliana AOX1a (At3g22370) is not significantly induced by cGMP neither after 2 nor 5 h19 and this observation concurs with our own findings21 whereas within the same timeframe PAL is (Fig. 1).
Figure 1.
Time-course of NO, H2O2, ET, SA and cGMP accumulation after fumigation with O3 in BelW3 tobacco. Gray arrows indicate established relationships between the signalling molecules, white arrows are proposed hypothetical links. The small arrows indicate the time of first significant accumulation of the respective molecules after fumigation with O3. The response to O3 includes a cascade of signal molecules leading to the expression of early and late defense genes. In this model early and NO responsive gene expression (AOX1a) does not depend on cGMP, while the late transcriptional response (e.g., of PAL) is at least in parts critically dependent on cGMP.
Outlook
We are currently performing an extended series of experiments that will resolve the temporal, spatial and stimulus specific induction patterns of O3 and NO dependent cGMP transients and link them to the cGMP dependent transcriptome and proteome. Based on both the available microarray data and our preliminary results, we hypothesise, that early (<3 hours) O3 and NO responsive gene expression including AOX1a does not depend on cGMP, while the late transcriptional response (>3 hours) is at least in parts critically dependent on cGMP. An example for a late response would be e.g., PAL (Fig. 1). In addition, we foresee that cGMP also has an important late role in responding to homeostatic disturbances caused by biotic and abiotic stresses. Such a role might require prolonged elevation of cytosolic cGMP levels—rather than short transients—which in turn can activate cGMP dependent ion channels. A function of cGMP in long-term ionic regulation after stress is also entirely compatible with the finding of cGMP dependent transcriptional activation of ion channel encoding genes.19
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/4818
References
- 1.Purvis A, Shewfelt R. Does the alternative pathway ameliorate chilling injury in sensitive plant tissue? Physiol Plant. 1993;88:712–718. doi: 10.1111/j.1399-3054.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 2.Wagner A, Krab K. The alternative respiration pathway in plants - Role and regulation. Physiol Plant. 1995;95:318–325. [Google Scholar]
- 3.Vanlerberghe G, McIntosh L. Mitochondrial electron transport regulation of nuclear gene expression: Studies with the alternative oxidase gene of tobacco. Plant Physiol. 1994;105:867–874. doi: 10.1104/pp.105.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhoads D, McIntosh L. Cytochrome and alternative pathway respiration in tobacco: Effects of salicylic acid. Plant Physiol. 1993;103:877–883. doi: 10.1104/pp.103.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytol. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang X, von Rad U, Durner J. Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta. 2002;215:914–923. doi: 10.1007/s00425-002-0828-z. [DOI] [PubMed] [Google Scholar]
- 7.Simons B, Millenaar F, Mulder L, Van Loon L, Lambers H. Enhanced expression and activation of the alternative oxidase during infection of Arabidopsis with Pseudomonas syringae pv tomato. Plant Physiol. 1999;120:529–538. doi: 10.1104/pp.120.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasqualini S, Della Torre G, Ferranti F, Ederli L, Piccioni C, Reale L, Antonielli M. Salicylic acid modulates ozone-induced hypersensitive cell death in tobacco plants. Plant Physiol. 2002;115:204–212. doi: 10.1034/j.1399-3054.2002.1150205.x. [DOI] [PubMed] [Google Scholar]
- 9.asqualini S, Piccioni C, Reale L, Ederli L, Della Torre G, Ferranti F. Ozone-induced cell death in tobacco cultivar BelW3 plants: The role of programmed cell death in lesion formation. Plant Physiol. 2003;133:1122–1134. doi: 10.1104/pp.103.026591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, Ferranti F, Tosti N, Pasqualini S. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozon-treated Tobacco plants. Plant Physiol. 2006;142:595–608. doi: 10.1104/pp.106.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasqualini S, Paolocci F, Borgogni A, Morettini R, Ederli L. The overexpression of an alternative oxidase gene triggers ozone sensitivity in tobacco plants. Plant Cell Environ. 2007;12:1545–1556. doi: 10.1111/j.1365-3040.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 12.Newton R, Roef L, Witters E, Van Onckelen H. Tansley review no. 106 - Cyclic nucleotides in higher plants: The enduring paradox. New Phytol. 1999;143:427–455. doi: 10.1046/j.1469-8137.1999.00478.x. [DOI] [PubMed] [Google Scholar]
- 13.Schaap P. Guanylyl cyclases across the tree of life. Front Biosci. 2005;10:1485–1498. doi: 10.2741/1633. [DOI] [PubMed] [Google Scholar]
- 14.Meier S, Gehring C. Emerging roles in plant biotechnology for the second messenger cGMP - Guanosine 3′, 5′-cyclic monophosphate. African Journal of Biotechnology. 2006;5:1687–1692. [Google Scholar]
- 15.Donaldson L, Ludidi N, Knight MR, Gehring C, Denby K. Salt and osmotic stress cause rapid increases in Arabidopsis thaliana cGMP levels. FEBS Lett. 2004;569:317–320. doi: 10.1016/j.febslet.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Durner J, Wendehenne D, Klessig D. Defense gene induction in Tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prado AM, Porterfield DM, Feijo JA. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development. 2004;131:2707–2714. doi: 10.1242/dev.01153. [DOI] [PubMed] [Google Scholar]
- 18.Delledonne M. NO news is good news for plants. Curr Opin Plant Biol. 2005;8:390–396. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Maathuis FJM. cGMP modulates gene transcription and cation transport in Arabidopsis roots. Plant J. 2006;45:700–711. doi: 10.1111/j.1365-313X.2005.02616.x. [DOI] [PubMed] [Google Scholar]
- 20.Hu X, Neill SJ, Tang Z, Cai W. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol. 2005;137:663–670. doi: 10.1104/pp.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tosti N, Pasqualini S, Borgogni A, Ederli L, Falistocco E, Crispi S, Paolocci F. Gene expression profiles of O3-treated Arabidopsis plants. Plant Cell Envion. 2006;29:1686–1702. doi: 10.1111/j.1365-3040.2006.01542.x. [DOI] [PubMed] [Google Scholar]