Abstract
It is now well-established that neuropilins (NRP1 and NRP2), first described as mediators of neuronal guidance, are also mediators of angiogenesis and tumor progression. NRPs are receptors for the class-3 semaphorin (SEMA) family of axon guidance molecules and also for the vascular endothelial growth factor (VEGF) family of angiogenic factors. VEGF-NRP interactions promote developmental angiogenesis as shown in mouse knockout and zebrafish knockdown studies. There is also evidence that NRPs mediate tumor progression. For example, overexpression of NRP1 enhances tumor growth whereas NRP1 antagonists, such as soluble NRP1 and anti-NRP1 antibodies, inhibit tumor growth. Furthermore, some class-3 SEMAs acting via NRPs inhibit tumor angiogenesis, progression and metastasis. Clinical data suggest that high NRP levels correlate with poor prognosis and survival in a variety of cancer types. Taken together, these results suggest that NRPs are potentially valuable targets for new anti-cancer therapies. We analyze here the current knowledge on NRPs and their role in angiogenesis and tumor progression and enumerate strategies for targeting these receptors.
Keywords: neuropilin, semaphorin, VEGF, angiogenesis, axon guidance, cancer, metastasis
Neuropilin Background
Anti-VEGF antibodies have received much attention lately for their ability to block tumor angiogenesis and prolong the life of cancer patients.1 In 2004, Bevacizumab (Avastin), a humanized monoclonal antibody against VEGF-A, became the first antiangiogenic drug approved by the FDA as a first line treatment for metastatic colorectal cancer in combination with chemotherapy. Another anti-VEGF antibody, Ranibizumab (Lucentis), a monoclonal antibody Fab, has been successful in the treatment of neovascularization associated with wet neovascular age-related macular degeneration (AMD), thereby alleviating blindness in patients.2 However, in cancer patients the anti-VEGF-chemotherapy combination has had adverse effects including hypertension, impaired wound healing and arterial thrombotic events.3 Thus, a promising development surfaced recently in an article by Pan et al.4 where it was reported that antibodies to neuropilin-1 (NRP1) in combination with anti-VEGF enhanced the ability of anti-VEGF to block tumor growth.
There are two NRPs, NRP1 and NRP2. These two NRPs are single pass transmembrane glycoproteins. NRPs were first discovered to be expressed in neurons.5 Subsequently, NRP1 was identified as the receptor for semaphorin 3A (SEMA3A), a mediator of axon guidance that repels axons and collapses growth cones.6 Surprisingly, NRPs were found soon thereafter to be receptors for the VEGF family of angiogenesis factors, suggesting that the receptor could be involved in blood vessel formation.7 Structurally, NRPs have a large extracellular domain (∼840 amino acid residues) containing the A, B and C subdomains, a short transmembrane domain (∼25 residues) and a ∼40 residue long cytoplasmic sequence (Fig. 1). VEGF165 binds to the B domain, whereas class-3 SEMAs bind to both the A and B domains.8–10 VEGF is a modular protein consisting of eight exons, whose differential splicing gives rise to different isoforms. Importantly, exon 4 binds to the high affinity VEGFR-2 tyrosine kinase receptor, whereas it is exons 7 and 8 that bind to NRPs (Fig. 2A). Thus, VEGF165 can bind simultaneously to two receptors, VEGFR-2 and NRP. VEGF165 stimulates enhanced chemotaxis in EC that express VEGFR-2 and NRP1 compared to EC expressing VEGFR-2 alone.7 Since NRPs do not have signaling motifs, it has been suggested that NRP1 is a coreceptor for VEGFR-2.
Figure 1.
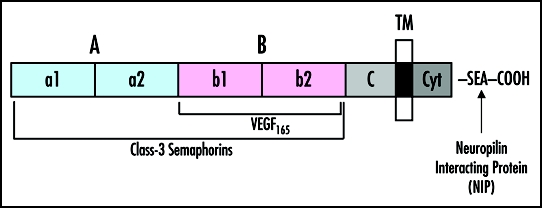
Schematic representation of the NRP domain structure. The sites of interaction with the respective extracellular ligands, VEGF165 and class-3 SEMAs, along with the intracellular binding partner, Neuropilin Interacting Protein (NIP), are shown.
Figure 2.
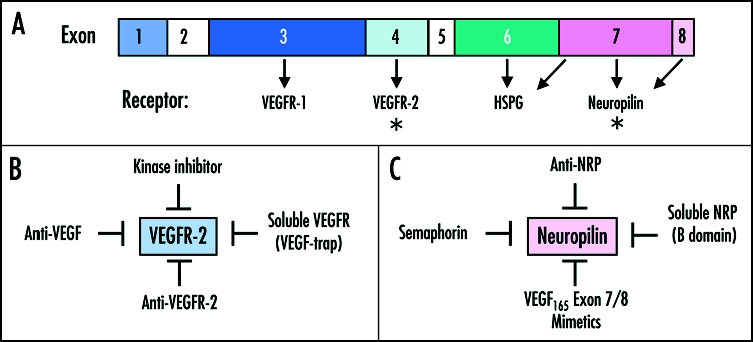
(A) Schematic view of the VEGF exon organization. Amino acid residues encoded by exons 3 and 4, respectively, are responsible for interaction with VEGFR-1 and VEGFR-2, respectively. Exons 6 and 7 represent the VEGF heparan sulphate proteoglycan (HSPG) binding domains. Exons 7 and 8 are involved in NRP binding. The asterisks represent the two major targets of therapeutics, (VEGFR-2 and NRP). (B) Strategies to inhibit VEGF165 signaling through VEGFR-2. These include VEGFR-2 kinase inhibitors (e.g., PTK787), soluble VEGFRs (e.g., VEGF-trap), antibodies against VEGFR-2 (e.g., DC101) and antibodies against VEGF (Bevacizumab, Ranibizumab). (C) Strategies to target VEGF165 interaction with neuropilins. These include: anti-NRP1 antibodies, soluble NRP isoforms and semaphorins (e.g., SEMA3A, SEMA3B, SEMA3F). In addition, there are a number of peptide-mimetics of VEGF165 exons 7/8 which block interaction with NRPs such as VEGF165 (137–160), A7R, EG3287, Tuftsin and its analog TKPPR (see Table 2).
Why is anti-VEGF combined with anti-NRP1 more effective as a tumor growth inhibitor than anti-VEGF alone? Presumably, VEGF165 exon 4 interactions with VEGFR-2 and VEGF165 exons 7/8 interactions with NRPs constitute different signaling pathways and therefore different targets. There is evidence that NRP signaling can be independent of VEGFR-2, by binding of NRP interacting protein (NIP), a PDZ domain-containing protein.11 NIP is identical to RGS-GAIP- interacting protein (GIPC), which has been suggested to be involved in vesicular trafficking.12 GIPC interacts with the NRP1 C-terminal residues, SEA-COOH. In zebrafish, GIPC knockdown with morpholinos resulted in a vascular defect resembling NRP1 knockdown.11 Another NIP is Synectin, which is required for arterial branching independent of VEGF/VEGFR-2.13,14
In the study by Pan et al. the properties of two anti-NRP antibodies were investigated, anti-NRP1A and anti-NRP1B, directed against the NRP1 A or B domains, respectively. Both anti-NRP1 antibodies inhibited VEGF165-induced EC migration, EC sprouting and neovascularization in the corneal pocket assay but did not inhibit VEGF165-induced VEGFR-2 phosphorylation or downstream signaling, EC proliferation, or vascular permeability. Anti-NRP1B was more effective, consistent with the B domain being the VEGF165 binding domain. Tumor blood vessels of mice treated with both anti-VEGF and anti-NRP1 demonstrated low pericyte association with tumor EC. Since pericytes stabilize tumor vessels, the lack of pericytes suggests that the tumor blood vessels are more fragile and susceptible to anti-angiogenesis therapy. Anti-NRP also inhibited vascular remodeling in the mouse-developing retina. Thus, anti-NRP1 antibodies might prevent tumor vessel maturation and, in this way, keep vessels dependent on VEGF and, thus, more susceptible to anti-VEGF treatment.
Neuropilins and Angiogenesis
What is the evidence that NRPs mediate angiogenesis, both normal and pathological? NRPs are expressed in the vascular system with some degree of vessel type specificity, in the mouse, chick, and zebrafish embryo. NRP1 is expressed in arterial EC whereas NRP2 is expressed in vein and lymphatic EC.15–18 Mouse knockout studies have been invaluable in determining the role of NRPs in blood vessel development. A number of animal models have been used to delineate NRP function in angiogenesis. The first indication that NRP1 was involved in angiogenesis was the demonstration that overexpression of Nrp1 in transgenic mice was embryonic-lethal and displayed vascular defects including excess numbers of blood vessels, dilated blood vessels, hemorrhage, and malformed hearts and limbs.19 Nrp-1-deficient mice died in utero at E12.5 to E13.5 and exhibited vascular defects including abnormal yolk sacs, abnormal neuronal vascularization, disorganized blood vessels, lack of normal branching, missing capillary networks, cardiovascular defects including agenesis of the bronchial arches, defects in the dorsal aorta, and transposition of aortic arches.20 Nrp2-deficient mice were viable,21 developed arteries and veins normally but displayed a severe reduction of small lymphatic vessels and capillaries.16 Double Nrp1/Nrp2 knockout mice died at E8.5 with a more severe vascular phenotype than Nrp1-deficient mice including avascular yolk sacs, growth retardation and lack of vessel development, capillary formation, and branching.22
In zebrafish, the knockdown of NRP1 by antisense morpholinos (MO) resulted in vascular defects.23 These defects included a loss of circulation via the intersegmental vessels (ISV), via the dorsal longitudinal anastomotic vessels (DLAV), and via the caudal vein plexus. The ISV correspond to angiogenic sprouts. Furthermore, abnormal direct connections between the artery and vein resembling fistulas resulted in aberrant return of blood circulation back to the heart. Of interest, MO treatment at the 1–4 cell stage did not inhibit axial vessel formation. Thus, in zebrafish, NRP1 is a regulator of angiogenesis but not vasculogenesis.
Neuropilins and Semaphorins
The other set of ligands for NRPs are the class-3 family of semaphorins (SEMA), of which there are seven members (A–G). SEMAs were first described as negative mediators of axon pathfinding that repel axons and collapse growth cones.6,24 Surprisingly, it turned out that some semaphorins are angiogenesis inhibitors as well. The SEMA-NRP complex alone does not transmit signals. Instead, NRPs form complexes with plexins, that are transmembrane proteins that act as substrates for kinases, and that transduce the SEMA signal.25,26 SEMA3A inhibits EC adhesion, migration and sprouting.27,28 Semaphorins also inhibit tumor growth, tumor angiogenesis and metastasis. Overexpression of SEMA3F in melanoma cells in vivo inhibited tumor angiogenesis and metastasis.29 Interestingly, it appeared that SEMA3F acted by repelling EC and preventing the invasion of tumor by blood vessels. This mechanism mimics the well-characterized SEMA repulsion of axons. SEMA3B also appears to be an inhibitor of tumor progression. Adenocarcinoma cells transfected with SEMA3B are less tumorigenic compared to controls.30 Another mechanism involves p53, a tumor suppressor. p53 increases the levels of SEMA3F,31 which in turn is also considered to be a tumor suppressor.32 p53 knockdowns show enhanced tumor angiogenesis concomitant with lower SEMA3F and NRP2 gene expression.31 It should be noted that SEMA3E is an exception to the rule. In contrast to other class-3 SEMAs, SEMA3E directly binds to Plexin-D1; SEMA3E-plexin D1 interactions are required for the patterning of intersomitic blood vessels (ISV) and do so in a NRP-independent way.33 In contrast with other SEMAs whose activity is suppressed by furin-dependent proteolysis,34 SEMA3E becomes activated by cleavage and in turn promotes lung metastasis.35
Importantly, SEMA3A and VEGF165 are competitive inhibitors. VEGF165 inhibits SEMA3A-induced dorsal root ganglia (DRG) collapse, whereas SEMA3A inhibits VEGF165-induced EC motility.28 The structural basis for this competitiveness may be that SEMAs and VEGF165 both bind to the NRP1/NRP2 B domain suggesting that they have some binding sites in common. Thus, the ratio of VEGF165 to SEMA might tip the balance between promoting and blocking angiogenesis. An evidence for this possibility is that a high VEGF165/SEMA ratio has been correlated with poor prognosis in ovarian carcinoma patients.36
Neuropilins and Tumors
NRP1-driven angiogenesis contributes to tumor growth in mice. For example, inducible overexpression of NRP1 in prostate carcinoma cells in vivo resulted in larger and highly vascular tumors.37 Tumor sections had high levels of VEGF165 protein, suggesting that VEGF165 was retained in the tumor by binding to NRP1. Similarly, human colon adenocarcinoma cells overexpressing NRP1 led to larger and more angiogenic tumors compared to controls.38 Overexpression of NRP1 in U87MG human glioblastoma, which expresses relative low levels of the receptor, resulted in accelerated tumor growth and angiogenesis compared to control tumors.39 On a different note, overexpression of NRP1 in pancreatic cancer cells renders these cells less susceptible to detachment-induced apoptosis (anoikis) and more resistant to chemotherapy (gemcitabine and 5-fluorouracil).40 However, opposite results in pancreatic cancer were obtained by Gray et al.41 In this study, overexpression of NRP1 led to decreased tumorigenity of a pancreatic cancer line and, by contrast, NRP1 silencing significantly increased tumorigenity.
Neuropilin Expression in Cancer Patients
Clinical samples derived from cancer patients have demonstrated that in many different varieties of tumors, NRP expression is elevated and that this enhancement is correlated with poor prognosis and survival (Table 1). In glioma, an increased NRP1 expression without concomitant increase in VEGF-A or VEGF receptors was reported.42 These patients had a poorer prognosis than those without NRP1 overexpression. Patients with newly diagnosed untreated acute myeloid leukemia (AML) demonstrated significant increase in NRP1 expression and significantly poorer 5-year overall survival compared to controls.43 Patients with advanced colorectal carcinoma and high levels of NRP1 staining showed significantly higher incidences of lymph node or liver metastasis and shorter survival than patients with low levels of NRP.44 In gastrointestinal cancers NRP1 expression correlated with increased invasive growth.45 NRP2 expression can also correlate with tumor progression. For example, osteosarcomas with NRP2 expression showed significantly increased vascularity and poorer prognosis.46 Expression of both NRP1 and NRP2 was significantly higher in ovarian carcinomas than in benign tumors. Interestingly, SEMA was strongly expressed in benign tumors and much less expressed in late stage carcinomas.36 Patients with a high VEGF to SEMA ratio showed poorer survival than those with low VEGF/SEMA ratios. The expression of NRP1 and NRP2 in nonsmall cell lung carcinomas (NSCLC) was higher compared to the expression in extraneoplastic tissue.47 Moreover, patients with coexpression of the two NRPs showed poorer prognosis and increased vessel counts compared to individuals without coexpression. In nonsmall cell lung carcinoma, the levels of NRP1 and NRP2 increased during different stages of lung cancer, progressing from dysplasia to microinvasive carcinoma.48 VEGF was also elevated whereas SEMA3F levels remained low, again showing an inverse relationship of VEGF and SEMA expression in cancer. Although these studies appear to be strong evidence for NRP expression being elevated in tumors, there is a contrary report indicating that expression of NRP1 contributes to a better prognosis in colon cancer.49 Gene expression levels of NRP1 in this tumor were decreased compared to the extra-neoplastic tissue levels. On the other hand, NRP2 expression was not diminished in these tumors.
Table 1.
Correlation between neuropilin expression and tumor progression
| Tumor Type | NRP1 | NRP2 | Status | Reference |
| Glioma | ↑ | n.a.1 | Poorer prognosis | 42 |
| Acute myeloid leukemia | ↑ | n.a. | Poorer survival | 43 |
| Colon cancer | ↑ | n.a. | Poorer survival, higher lymph node and liver metastasis | 44 |
| Gastrointestinal cancer | ↑ | n.a. | Higher invasiveness | 45 |
| Osteosarcoma | n.a. | ↑ | Increased vascularity, poorer prognosis | 46 |
| Ovarian carcinoma | ↑ | ↑ | Poorer prognosis; high VEGF/SEMA ratio is prognostic for poor survival | 36 |
| Nonsmall cell lung carcinoma (NSCLC) | ↑ | ↑ | Poorer prognosis; increased vascularity | 47 |
| Nonsmall cell lung carcinoma (NSCLC) | ↑ | ↑ | NRP1, NRP2, and VEGF165 increase with tumor stage; loss of SEMA3F in premalignant lesions | 48 |
| Colon cancer | ↓ | n.c.2 | Poorer prognosis | 49 |
not analyzed;
no change.
Neuropilin Antagonists
Antagonists of VEGF-VEGF receptor interactions have shown promise as inhibitors of tumor angiogenesis. These antagonists include anti-VEGF antibodies (Bevacizumab and Ranimizumab, Genentech),50 anti-VEGFR-2 antibody (DC101),51 VEGR kinase inhibitors (e.g., PTK787)52 and soluble VEGFRs, such as VEGF-trap, a soluble receptor consisting of the second Ig domain of VEGFR-1 fused to the third Ig domain of VEGFR-2 (Fig. 2B).53
The efficacy of anti-NRP1 antibody as an inhibitor of tumor growth in a preclinical tumor model4 suggests that it could be worthwhile to develop other strategies for inhibiting NRP activity. A number of NRP antagonists have been reported. These antagonists include anti-NRP1 antibodies (described above), semaphorins (described above), soluble NRP1 (sNRP1), and peptides derived from VEGF165 and from NRPs that block VEGF165-NRP interactions (Fig. 2C and Table 2).
Table 2.
Anti-neuropilin strategies
| Factor | Mechanism | Reference |
| Semaphorins | Competition for VEGF165 binding to NRPs; Repulsion of EC (SEMA3F) | 28; 29 |
| Anti-NRP antibody | Inhibition of VEGF165 binding to full-length NRP; Additive effect with anti-VEGF in tumor growth inhibition | 4 |
| Soluble NRP | Inhibition of VEGF165 signaling; antitumor activity | 55; 56 |
| NRP B domain | Inhibition of VEGF165 binding to full-length NRP | 8 |
| VEGF165 (137–160)1 | Inhibition of VEGF-induced EC proliferation | 58 |
| ATWLLPR (A7R)2 | Inhibition of VEGF165 binding to NRP; Antiangiogenic and antitumor activity | 61 |
| EG32873 | Inhibition of VEGFR-2 phosphorylation and downstream signaling | 59 |
| TKPPR4 | Inhibition of VEGFR-2 activation | 60 |
VEGF165 fragment consisting of exon 7 (amino acids 22–44) and the first amino acid of exon 8, a cysteine residue;
Peptide identified by screening a mutated phage library against an anti-VEGF antibody that blocks VEGF165-dependent EC proliferation;
Byciclic peptide consisting of exon 7 (amino acids 23–4) and exon 8;
Tuftsin-like peptide (TKPR). Homologous to VEGF165 exon 8.
Naturally occurring sNRPs originate from alternative splicing of the NRP gene within introns yielding several spliced isoforms.54,55 At the protein level sNRPs consist of the extracellular NRP domains, in particular the A and B subdomains.54–56 Overexpression of sNRP1 in prostate cancer cells resulted in tumors that were severely apoptotic and contained mainly damaged and hemorrhagic tumor vessels, ostensibly by inhibiting VEGF function.55 Thus, sNRP1 may be acting as a VEGF trap. Similarly, sNRP1 inhibited human breast carcinoma cell migration by a mechanism implying sequestering of endogenous VEGF165.56 Taken together, these data suggest that NRP soluble isoforms might act as VEGF165 antagonists that sequester VEGF165 and prevent the tumorigenic and angiogenic effects mediated by the full-length NRP.
VEGF165 via its exons 7/8 binds solely to the NRP B domain (Geretti E and Klagsbrun M, unpublished).8,9,57 Thus, a NRP B domain peptide is a potential competitive inhibitor of VEGF-NRP interactions. An advantage of the NRP B domain peptide would be that it targets VEGF165 only, and not the SEMAs, which require both the A and B domains for optimal interaction with receptor (Geretti E and Klagsbrun M, unpublished).9,10 This selectivity might be important for in vivo anti-angiogenesis applications where it would be desirable to inhibit VEGF activity but not semaphorins, which are angiogenesis inhibitors.
Strategies have been developed to target VEGF-NRP1 interaction based on interfering with VEGF exons 7 and 8 binding to NRP1. For example, a peptide corresponding to VEGF exon 7 (44 amino acids) and 8 (6 amino acids) inhibited VEGF165 binding to NRP1 and VEGF165-induced EC proliferation.58 The active region was found to be in the second half of exons 7, amino acids 22–44 plus the first amino acid of exon 8, a cysteine residue. A bicyclic peptide, EG3287, based on a NRP1 binding site located in VEGF exons 7 and 8, inhibited VEGF165 binding to PAE-NRP1 cells but not to PAE cells expressing VEGF receptor tyrosine kinases (VEGFR-1 and VEGFR-2).59 The bicyclic peptide, however, did inhibit cross-linking of VEGF165 to VEGFR-2 in ECs coexpressing VEGFR-2 and NRP1, and as a result inhibited VEGF165 signaling, e.g., VEGFR-2, PLC-γ and ERK phosphorylation.59 The importance of VEGF exon 8 for NRP binding was further substantiated by a study showing that Tuftsin (TKPR), an analogue of VEGF exon 8 (CDKPRR), inhibited VEGF165 binding to NRP1.60 An analogous synthetic peptide, TKPPR, was even a higher affinity antagonist. TKPPR inhibited VEGF165-induced VEGFR-2 activation without directly inhibiting VEGF165 binding to VEGFR-2. Another peptide, ATWLLPR (A7R), inhibited VEGF165 binding to NRP1 but not to VEGFR-2. A7R inhibited EC proliferation, tube formation and growth of MDA-MB231 mouse xenografts.61 Taken together it appears that a class of VEGF165 inhibitors has been developed that consists of rationally designed peptides for the purpose of blocking VEGF-NRP interactions.
Future Directions for NRP Research
There is solid evidence that NRPs mediate tumor progression, either as stimulators in VEGF-mediated pathways or as inhibitors in SEMA-mediated pathways. Several areas of research might prove to be beneficial. One would be to develop more NRP antagonists in a rational manner. For example, anti-NRP1 antibody has proven to be effective in inhibiting tumor growth in combination with anti-VEGF antibody. An anti-NRP2 antibody could prove to be useful in curbing tumor progression in combination with anti-NRP1 antibody. An anti-NRP2 antibody might be useful in inhibiting lymphangiogenesis in tumors since lymphatic EC express solely NRP2.
Besides the antibody approach, another strategy that is being developed and holds promise is to target VEGF exon 7 interactions with the NRP B domain. NRP soluble fragments and VEGF exons 7/8 mimetics that do so have been described and these studies could be expanded. The availability of B domain crystal structures, for example, the b1 domain of NRP162 and the B domain of NRP1, cocrystallized with Tuftsin,63 offer valuable starting points for rational structure-based inhibitor design. For example, mutations in the B domain that would bind VEGF more tightly might be efficient in sequestering VEGF (Geretti E and Klagsbrun M, unpublished). In all of these strategies, it would be important not to affect adversely SEMA binding to NRPs since some SEMAs are inhibitors of tumor progression.
Another important aspect of NRP relationship to tumors would be to expand analysis of NRPs expression along with VEGF and SEMAs levels in patients. So far, it has been found that at least seven tumor types show an increase in NRP1, NRP2 or both NRP levels. A larger sampling of patient data would be critical in ascertaining reliability. Most of this work is based on immunohistological analysis of tumor sections but it would be beneficial to develop more quantitative analytical methods (e.g., ELISA) for this purpose. An interesting possibility is that the VEGF/SEMA ratios might be diagnostic. Finding ways of upregulating endogenous SEMAs (3A, 3B and 3F) might counteract VEGF activity.
In summary, NRPs can mediate either the stimulation (VEGF) or the inhibition (SEMA) of tumor angiogenesis and progression. Inhibiting VEGF-NRP interactions while promoting SEMA-NRP interactions might be rational approaches to regulating tumor angiogenesis and progression.
Acknowledgments
We thank Drs. Dhara Amin, Silvia Coma and Kashi Javaherian for critical evaluation of the manuscript. We thank Melissa Herman for proofreading the manuscript and Kristin Johnson for technical assistance in figure preparation. This work was supported by National Institute of Health NCI grants CA37392 and CA45548 (to Michael Klagsbrun).
Abbreviations
- NRP
neuropilin
- SEMA
semaphorin
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
- EC
endothelial cell
- PAE cells
porcine aortic endothelial cells
- ISV
intersegmental vessels
- DRG
dorsal root ganglia
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/4490
References
- 1.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: Safety profile and management of adverse events. Semin Oncol. 2006;33:S26–S34. doi: 10.1053/j.seminoncol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le Couter J, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Takagi S, Tsuji T, Amagai T, Takamatsu T, Fujisawa H. Specific cell surface labels in the visual centers of Xenopus laevis tadpole identified using monoclonal antibodies. Dev Biol. 1987;122:90–100. doi: 10.1016/0012-1606(87)90335-6. [DOI] [PubMed] [Google Scholar]
- 6.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 7.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 8.Mamluk R, Gechtman Z, Kutcher ME, Gasiunas N, Gallagher J, Klagsbrun M. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem. 2002;277:24818–24825. doi: 10.1074/jbc.M200730200. [DOI] [PubMed] [Google Scholar]
- 9.Gu C, Limberg BJ, Whitaker GB, Perman B, Leahy DJ, Rosenbaum JS, Ginty DD, Kolodkin AL. Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165. J Biol Chem. 2002;277:18069–18076. doi: 10.1074/jbc.M201681200. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura F, Tanaka M, Takahashi T, Kalb RG, Strittmatter SM. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21:1093–1100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Mukhopadhyay D, Xu X. C terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. Faseb J. 2006;20:1513–1515. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- 12.Cai H, Reed RR. Cloning and characterization of neuropilin-1-interacting protein: A PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci. 1999;19:6519–6527. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y, Li M, Chen W, Simons M. Synectin, syndecan-4 cytoplasmic domain binding PDZ protein, inhibits cell migration. J Cell Physiol. 2000;184:373–379. doi: 10.1002/1097-4652(200009)184:3<373::AID-JCP12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Chittenden TW, Claes F, Lanahan AA, Autiero M, Palac RT, Tkachenko EV, Elfenbein A, Ruiz de Almodovar C, Dedkov E, Tomanek R, Li W, Westmore M, Singh JP, Horowitz A, Mulligan-Kehoe MJ, Moodie KL, Zhuang ZW, Carmeliet P, Simons M. Selective regulation of arterial branching morphogenesis by synectin. Dev Cell. 2006;10:783–795. doi: 10.1016/j.devcel.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev. 2001;109:115–119. doi: 10.1016/s0925-4773(01)00518-4. [DOI] [PubMed] [Google Scholar]
- 16.Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 17.Eichmann A, Yuan L, Moyon D, Lenoble F, Pardanaud L, Breant C. Vascular development: From precursor cells to branched arterial and venous networks. Int J Dev Biol. 2005;49:259–267. doi: 10.1387/ijdb.041941ae. [DOI] [PubMed] [Google Scholar]
- 18.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 19.Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121:4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Bagri A, Zupicich JA, Zou Y, Stoeckli E, Pleasure SJ, Lowenstein DH, Skarnes WC, Chedotal A, Tessier-Lavigne M. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron. 2000;25:43–56. doi: 10.1016/s0896-6273(00)80870-3. [DOI] [PubMed] [Google Scholar]
- 22.Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci USA. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee P, Goishi K, Davidson AJ, Mannix R, Zon L, Klagsbrun M. Neuropilin-1 is required for vascular development and is a mediator of VEGF-dependent angiogenesis in zebrafish. Proc Natl Acad Sci USA. 2002;99:10470–10475. doi: 10.1073/pnas.162366299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura F, Kalb RG, Strittmatter SM. Molecular basis of semaphorin-mediated axon guidance. J Neurobiol. 2000;44:219–229. doi: 10.1002/1097-4695(200008)44:2<219::aid-neu11>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Tamagnone L, Comoglio PM. Signalling by semaphorin receptors: Cell guidance and beyond. Trends Cell Biol. 2000;10:377–383. doi: 10.1016/s0962-8924(00)01816-x. [DOI] [PubMed] [Google Scholar]
- 27.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Puschel AW, Bussolino F. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 28.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: Functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest. 2004;114:1260–1271. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tse C, Xiang RH, Bracht T, Naylor SL. Human Semaphorin 3B (SEMA3B) located at chromosome 3p21.3 suppresses tumor formation in an adenocarcinoma cell line. Cancer Res. 2002;62:542–546. [PubMed] [Google Scholar]
- 31.Futamura M, Kamino H, Miyamoto Y, Kitamura N, Nakamura Y, Ohnishi S, Masuda Y, Arakawa H. Possible role of semaphorin 3F, a candidate tumor suppressor gene at 3p21.3, in p53-regulated tumor angiogenesis suppression. Cancer Res. 2007;67:1451–1460. doi: 10.1158/0008-5472.CAN-06-2485. [DOI] [PubMed] [Google Scholar]
- 32.Sekido Y, Bader S, Latif F, Chen JY, Duh FM, Wei MH, Albanesi JP, Lee CC, Lerman MI, Minna JD. Human semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc Natl Acad Sci USA. 1996;93:4120–4125. doi: 10.1073/pnas.93.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 34.Adams RH, Lohrum M, Klostermann A, Betz H, Puschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. Embo J. 1997;16:6077–6086. doi: 10.1093/emboj/16.20.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christensen C, Ambartsumian N, Gilestro G, Thomsen B, Comoglio P, Tamagnone L, Guldberg P, Lukanidin E. Proteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasis. Cancer Res. 2005;65:6167–6177. doi: 10.1158/0008-5472.CAN-04-4309. [DOI] [PubMed] [Google Scholar]
- 36.Osada R, Horiuchi A, Kikuchi N, Ohira S, Ota M, Katsuyama Y, Konishi I. Expression of semaphorins, vascular endothelial growth factor, and their common receptor neuropilins and alleic loss of semaphorin locus in epithelial ovarian neoplasms: Increased ratio of vascular endothelial growth factor to semaphorin is a poor prognostic factor in ovarian carcinomas. Hum Pathol. 2006;37:1414–1425. doi: 10.1016/j.humpath.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. Faseb J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- 38.Parikh AA, Fan F, Liu WB, Ahmad SA, Stoeltzing O, Reinmuth N, Bielenberg D, Bucana CD, Klagsbrun M, Ellis LM. Neuropilin-1 in human colon cancer: Expression, regulation, and role in induction of angiogenesis. Am J Pathol. 2004;164:2139–2151. doi: 10.1016/S0002-9440(10)63772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu B, Guo P, Bar-Joseph I, Imanishi Y, Jarzynka MJ, Bogler O, Mikkelsen T, Hirose T, Nishikawa R, Cheng SY. Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene. 2007 doi: 10.1038/sj.onc.1210348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wey JS, Gray MJ, Fan F, Belcheva A, McCarty MF, Stoeltzing O, Somcio R, Liu W, Evans DB, Klagsbrun M, Gallick GE, Ellis LM. Overexpression of neuropilin-1 promotes constitutive MAPK signalling and chemoresistance in pancreatic cancer cells. Br J Cancer. 2005;93:233–241. doi: 10.1038/sj.bjc.6602663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gray MJ, Wey JS, Belcheva A, McCarty MF, Trevino JG, Evans DB, Ellis LM, Gallick GE. Neuropilin-1 suppresses tumorigenic properties in a human pancreatic adenocarcinoma cell line lacking neuropilin-1 coreceptors. Cancer Res. 2005;65:3664–3670. doi: 10.1158/0008-5472.CAN-04-2229. [DOI] [PubMed] [Google Scholar]
- 42.Osada H, Tokunaga T, Nishi M, Hatanaka H, Abe Y, Tsugu A, Kijima H, Yamazaki H, Ueyama Y, Nakamura M. Overexpression of the neuropilin 1 (NRP1) gene correlated with poor prognosis in human glioma. Anticancer Res. 2004;24:547–552. [PubMed] [Google Scholar]
- 43.Kreuter M, Woelke K, Bieker R, Schliemann C, Steins M, Buechner T, Berdel WE, Mesters RM. Correlation of neuropilin-1 overexpression to survival in acute myeloid leukemia. Leukemia. 2006;20:1950–1954. doi: 10.1038/sj.leu.2404384. [DOI] [PubMed] [Google Scholar]
- 44.Ochiumi T, Kitadai Y, Tanaka S, Akagi M, Yoshihara M, Chayama K. Neuropilin-1 is involved in regulation of apoptosis and migration of human colon cancer. Int J Oncol. 2006;29:105–116. [PubMed] [Google Scholar]
- 45.Hansel DE, Wilentz RE, Yeo CJ, Schulick RD, Montgomery E, Maitra A. Expression of neuropilin-1 in high-grade dysplasia, invasive cancer, and metastases of the human gastrointestinal tract. Am J Surg Pathol. 2004;28:347–356. doi: 10.1097/00000478-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Handa A, Tokunaga T, Tsuchida T, Lee YH, Kijima H, Yamazaki H, Ueyama Y, Fukuda H, Nakamura M. Neuropilin-2 expression affects the increased vascularization and is a prognostic factor in osteosarcoma. Int J Oncol. 2000;17:291–295. doi: 10.3892/ijo.17.2.291. [DOI] [PubMed] [Google Scholar]
- 47.Kawakami T, Tokunaga T, Hatanaka H, Kijima H, Yamazaki H, Abe Y, Osamura Y, Inoue H, Ueyama Y, Nakamura M. Neuropilin 1 and neuropilin 2 coexpression is significantly correlated with increased vascularity and poor prognosis in nonsmall cell lung carcinoma. Cancer. 2002;95:2196–2201. doi: 10.1002/cncr.10936. [DOI] [PubMed] [Google Scholar]
- 48.Lantuejoul S, Constantin B, Drabkin H, Brambilla C, Roche J, Brambilla E. Expression of VEGF semaphorin SEMA3F, and their common receptors neuropilins NP1 and NP2 in preinvasive bronchial lesions, lung tumours, and cell lines. J Pathol. 2003;200:336–347. doi: 10.1002/path.1367. [DOI] [PubMed] [Google Scholar]
- 49.Kamiya T, Kawakami T, Abe Y, Nishi M, Onoda N, Miyazaki N, Oida Y, Yamazaki H, Ueyama Y, Nakamura M. The preserved expression of neuropilin (NRP) 1 contributes to a better prognosis in colon cancer. Oncol Rep. 2006;15:369–373. [PubMed] [Google Scholar]
- 50.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prewett M, Huber J, Li Y, Santiago A, O'Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, Bohlen P, Hicklin DJ. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 52.Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O'Reilly T, Persohn E, Rosel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–2189. [PubMed] [Google Scholar]
- 53.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossignol M, Gagnon ML, Klagsbrun M. Genomic organization of human neuropilin-1 and neuropilin-2 genes: Identification and distribution of splice variants and soluble isoforms. Genomics. 2000;70:211–222. doi: 10.1006/geno.2000.6381. [DOI] [PubMed] [Google Scholar]
- 55.Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, Klagsbrun M. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc Natl Acad Sci USA. 2000;97:2573–2578. doi: 10.1073/pnas.040337597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cackowski FC, Xu L, Hu B, Cheng SY. Identification of two novel alternatively spliced Neuropilin-1 isoforms. Genomics. 2004;84:82–94. doi: 10.1016/j.ygeno.2004.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karpanen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, Tamagnone L, Alitalo K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. Faseb J. 2006;20:1462–1472. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- 58.Soker S, Gollamudi-Payne S, Fidder H, Charmahelli H, Klagsbrun M. Inhibition of vascular endothelial growth factor (VEGF)-induced endothelial cell proliferation by a peptide corresponding to the exon 7-encoded domain of VEGF165. J Biol Chem. 1997;272:31582–31588. doi: 10.1074/jbc.272.50.31582. [DOI] [PubMed] [Google Scholar]
- 59.Jia H, Bagherzadeh A, Hartzoulakis B, Jarvis A, Lohr M, Shaikh S, Aqil R, Cheng L, Tickner M, Esposito D, Harris R, Driscoll PC, Selwood DL, Zachary IC. Characterization of a bicyclic peptide neuropilin-1 (NP-1) antagonist (EG3287) reveals importance of vascular endothelial growth factor exon 8 for NP-1 binding and role of NP-1 in KDR signaling. J Biol Chem. 2006;281:13493–13502. doi: 10.1074/jbc.M512121200. [DOI] [PubMed] [Google Scholar]
- 60.von Wronski MA, Raju N, Pillai R, Bogdan NJ, Marinelli ER, Nanjappan P, Ramalingam K, Arunachalam T, Eaton S, Linder KE, Yan F, Pochon S, Tweedle MF, Nunn AD. Tuftsin binds neuropilin-1 through a sequence similar to that encoded by exon 8 of vascular endothelial growth factor. J Biol Chem. 2006;281:5702–5710. doi: 10.1074/jbc.M511941200. [DOI] [PubMed] [Google Scholar]
- 61.Starzec A, Vassy R, Martin A, Lecouvey M, Di Benedetto M, Crepin M, Perret GY. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life Sci. 2006;79:2370–2381. doi: 10.1016/j.lfs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Lee CC, Kreusch A, McMullan D, Ng K, Spraggon G. Crystal structure of the human neuropilin-1 b1 domain. Structure. 2003;11:99–108. doi: 10.1016/s0969-2126(02)00941-3. [DOI] [PubMed] [Google Scholar]
- 63.Vander Kooi CW, Jusino MA, Perman B, Neau DB, Bellamy HD, Leahy DJ. Structural basis for ligand and heparin binding to neuropilin B domains. Proc Natl Acad Sci USA. 2007;104:6152–6157. doi: 10.1073/pnas.0700043104. [DOI] [PMC free article] [PubMed] [Google Scholar]


