Abstract
Semaphorins are a large family of secreted and membrane-bound molecules initially implicated in the development of the nervous system and in axon guidance. More recently, they have been found to regulate cell adhesion and cell motility, angiogenesis, immune function and tumor progression. Notably, Semaphorins have been implicated with opposite functions in cancer: either as putative tumor suppressors and anti-angiogenic factors, or as mediating tumor angiogenesis, invasion and metastasis. Interestingly, Semaphorins may display divergent activities in different cell types. These multifaceted functions may be explained by the involvement of different kinds of semaphorin receptor complexes, and by the consequent activation of multiple signaling pathways, in different cells or different functional stages. Semaphorin signaling is largely mediated by the Plexins. However, semaphorin receptor complexes may also include Neuropilins and tyrosine kinases implicated in cancer. In this review, we will focus on major open questions concerning the potential role of Semaphorin signals in cancer.
Key words: semaphorin, plexin, neuropilin, migration, tumor, metastasis, signaling
Over twenty different Semaphorin genes are known in vertebrates. They were initially discovered as repelling cues for axons, in the wiring of the neural system. However, they are currently considered versatile signals regulating cell migration, angiogenesis, tissue morphogenesis, immune function and cancer.1–2 Semaphorins have been implicated with opposite functions in tumor progression (summarized in Fig. 1). For example, Semaphorins 3B and 3F are putative tumor suppressors, while the expression of Semaphorin 3C, 3E and 5C has been associated with tumor invasion and metastasis. Interestingly, certain Semaphorins display divergent activities in different cell types. These varied functions of Semaphorins are likely to be explained by the involvement of different receptor complexes and multiple signaling pathways.
Figure 1.
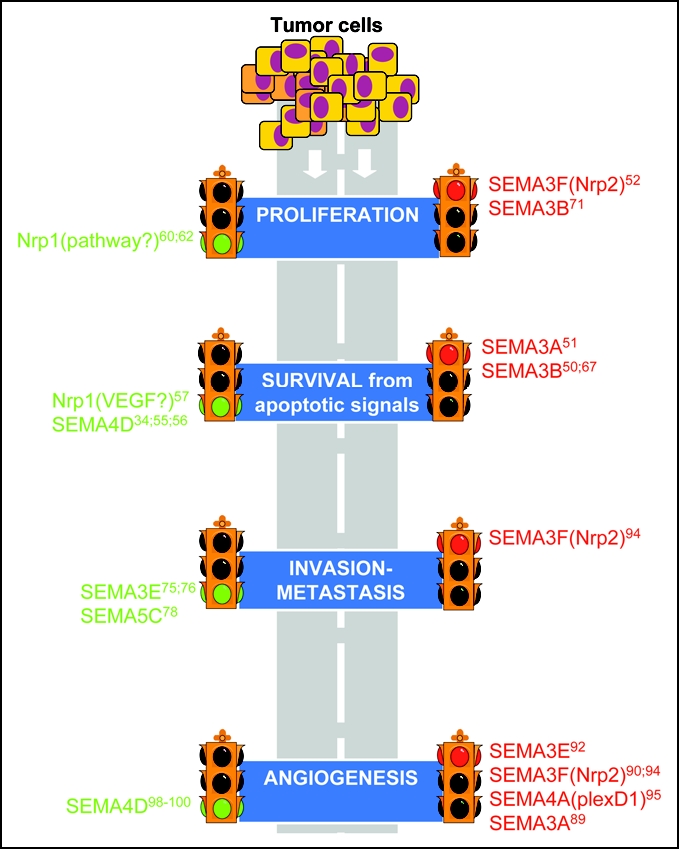
Semaphorin signals on the road to cancer invasion and metastasis. Semaphorins play a regulatory role on the main elements driving cancer progression. They can be seen as “stop” or “go” signals for tumor cells, as well as for stromal cells in the tumor microenvironment. The scheme features some examples of the semaphorin signals implicated so far. More information on the implicated receptors and functional activities of the different semaphorins are summarized in Table 1.
Semaphorin Signaling Pathways
Plexins are the high affinity receptors for Semaphorins, although many class 3 secreted Semaphorins require coreceptor molecules, the Neuropilins, to trigger Plexin-mediated signals.3 Nine Plexins and two Neuropilins are found in humans. In addition to Plexins and Neuropilins, other cell surface molecules have been reported to interact with the Semaphorins with lower affinity, and to mediate their signals via partly understood mechanisms.4–6
The intracellular region of the Plexins is highly conserved within the family but it does not share striking homology with other proteins or functional domains. It includes: (a) two highly conserved domains containing short motifs with similarity to GTPase Activating Proteins (GAP-like domains), reported to bind and inactivate R-Ras;7 (b) one “linker” domain, which interacts with GTP-bound monomeric GTPases of the Rho family but mediates no GAP activity;8,9 (c) in addition, Plexins of B subfamily include a C-terminal consensus sequence that associates with PDZ domains, and with PDZ-RhoGEFs in particular (inter alia, ref. 10). Several questions regarding the roles of plexin cytoplasmic domains remain to be answered. Are the GAP-like motifs only required to downregulate R-Ras activity, or do they have additional functions? Is the function of the linker domains diverse in the various Plexins? What is the specific functional relevance of the PDZ-domain binding sequence only found in B-subfamily Plexins? Notably, many additional intracellular signal transducers have been reported to associate with Plexins, although the structural requirements for these interactions and their regulatory mechanisms are largely unknown. Therefore, further structure-function studies are needed to identify the domains required to mediate different Semaphorin activities in vitro and in vivo.
Neuropilin-1 and Neuropilin-2 (Nrp1 and Nrp2) are Plexin-associated coreceptors for most secreted class 3 Semaphorins in vertebrates. In addition, they play an important role in association with VEGF-Receptors, whereby they regulate angiogenesis and lymphangiogenesis by binding VEGF family members.11–13 Notably, Nrp1-/- knock-out mice show embryonic lethality due to dramatic vascular defects, while Nrp2-/- knock-out mice are viable and show defects in lymphatic system formation.14–15 There is a competition between class 3 Semaphorins and VEGF165 (but not the 121 isoform) for the binding site on Nrp1, but it is less clear whether this is also true for Nrp2. However, many evidences seem to point against the idea that Semaphorins purely act as VEGF antagonists, and instead suggest that Semaphorin-mediated control of angiogenesis requires Plexin signalling.16–22 Moreover, certain results seem to break the dogma of Neuropilins only acting as coreceptors, and indicate that, upon binding VEGF/Semaphorins, they could elicit a signalling pathway on their own, via their short cytoplasmic domain and as yet largely unknown associated signal transducers.23 For instance, certain PDZ domain-containing proteins have been shown to associate with the C-terminus of NP-1, although the functional role of these interactions is unclear.24,25 Notably, the cytoplasmic tails of Nrp1 and Nrp2 are only 55% identical,26 which raises a major question: can they interact with different adaptors or signal transducers? Moreover, do the two Neuropilins have complementary roles or rather independent/antagonistic functions? Although, Nrp1 and Nrp2 have been shown to associate in receptor complexes upon overexpression,27 the functional relevance of this is not known. Furthermore, soluble forms of Nrp1 released in the extracellular space have been described, and they were found to act either as VEGF traps, or as pro-angiogenenic factors in different reports, likely dependent on the different structure of these truncated forms.28–32
It has been further shown that Plexins can associate in complexes with Tyrosine Kinase Receptors, such as ErbB2, VEGFR2, OTK, Met, and Ron.33–36 For example, Semaphorin 4D/PlexinB1 signalling may inhibit the migration of certain cancer cells, but it seems to have a reverse effect on others, when plexin-associated tyrosine kinases get transactivated in the complex. Furthermore, PlexinA1 can alternatively transduce Semaphorin 6D signals either in complex with KDR or with OTK tyrosine kinases, leading to opposite functions (invasive growth or cell repulsion, respectively) in different cell populations during myocardial development.36 The molecular mechanisms controlling these multimeric receptor complexes are poorly understood. For instance, it is not known whether this is exclusively regulated at the protein expression level, or adaptor molecules are required for their formation on the cell surface. It is possible that different components of the Semaphorin receptor complexes are diversely expressed in different phases of tumor progression and invasive growth, thereby leading to the formation of signalling complexes eliciting differential (and potentially antagonistic) pathways.
The signaling cascade of Plexins might also depend on their localization on the cell surface, possibly controlling a differential access to signal transducers. In fact, biological membranes contain specific microdomains, such as lipid rafts, which have been suggested to play a role in a variety of physiological and pathological processes. It is not known if the subcellular localization of resting and ligand-activated Plexins (and associated receptor complexes) is regulated and may have a functional relevance, for example, in determining and maintaining cell movement and directionality. Intriguingly, because lipid rafts are enriched in GPI-anchored proteins,37 class 7 Semaphorins (which are GPI anchored) might be preferentially located in these microdomains, although this remains to be studied.
In addition to mediating signals in receptor-expressing cells, transmembrane Semaphorins have been suggested to mediate so-called “reverse” signaling pathways via their intracellular domain. The strongest evidence that Semaphorins can trigger bidirectional signals was obtained for Semaphorin 6D/PlexinA1 interaction in cardiac development in chick embryo.38 The potential functional relevance of this mechanism for other Semaphorins needs further investigation. Moreover, can the extracellular domain of other Semaphorin receptors in addition to Plexins act as a ligand to induce reverse signalling in transmembrane semaphorins?
Semaphorin-Mediated Activities
In addition to their role in axon guidance, Semaphorins provide signals to regulate cell migration. Migrating cells are guided by the complex integration of multiple motility-promoting, motility-inhibiting and directional signals. Moreover, recent evidences indicate that tumor cell migration may occur in three different ways: mesenchymal, proteolysis-independent ameboid, and mesenchymal-ameboid transition modes.39–40 The mesenchymal migration mode is most commonly observed during development.41 It is characterized by elongated cells with established polarity, featuring a “leading” and a “trailing” edge. Leading edge advancement requires F-actin polymerization to induce cell protrusions,42 which in turn depends on Integrin-mediated adhesion to the ECM and on the activity of intracellular transducers connecting adhesive complexes with the actin cytoskeleton (such as monomeric GTPases). Moreover, this process often implies the release of metallo-proteases at the leading edge, to degrade extracellular matrix barriers. Notably, the leading edge contains a higher concentration of receptors for guiding cues (either attracting or repelling), and by integrating these signals it finely tunes the direction of migration. Semaphorins and plexins are known to be major regulators in this process.
Interestingly, it was found that tumor cells can also migrate with ameboid mode, independent of ECM degrading activity, via Rho kinase (ROCK)-dependent actin cytoskeleton remodeling and rounded cell morphology.40 The ability of tumor cells to switch between different migration modes, in response to environmental changes, is probably responsible for the limited efficacy of therapeutic agents aimed at inhibiting cancer invasion. It is currently thought that the main role of Semaphorin signals in cell migration is in the regulation of integrin function and actin dynamics at the leading edge, mechanisms required for mesenchymal-type of migration.22,43–45 The negative regulation of β1-Integrins mediated by plexins may thus hamper cancer cell migration and invasive potential. However, it could also cause cells to switch to ameboid movement, which is not dependent on strong matrix adhesions.46 It will be interesting to address this question experimentally by studying tumor cell migration/invasion in 3D gels of extracellular matrix.47 Notably, semaphorin-mediated activation of Tyrosine Kinase Receptors associated with plexins can instead lead to Rac activation and promote mesenchymal-like cell motility. Moreover, Semaphorin 3C, which is overexpressed in certain tumor cells, was reported to increase integrin-mediated adhesion, via as yet unclear mechanisms.48,49 Furthermore, it has been shown that Sema7A, a semaphorin bound to the cell surface with a GPI anchor and containing an RGD adhesive motif, is capable of activating β1-Integrin signalling in a plexin-independent manner (probably acting as pseudo-adhesive substrate) and promoting axonal outgrowth.4 Therefore semaphorins can regulate cell-substrate adhesion in several ways.
In addition to controlling cell migration, Semaphorins and their receptors have also been implicated in regulating cell proliferation, cell survival and differentiation. For example, Semaphorin 3A and Semaphorin 3B may act as VEGF165 antagonists and thereby lead to cell growth inhibition or apoptosis,50–51 Semaphorin 3F has been shown to have anti-proliferative activity,52–54 while Sema4D appears to be a pro-survival factor.34,55–56 Intriguingly, Neuropilin-1 overexpression has been reported to promote proliferation and prevent apoptosis in different tumor cell lines.57–62 Of potential relevance to cancer, is the reported function of Semaphorin6D-PlexinA1 signalling in the differentiation of dendritic cells and osteoclasts.63 Moreover, a subset of semaphorins have been clearly shown to regulate the immune function.64
Semaphorins and Semaphorin Receptors in Cancer
Cancer is a genetic disease, as specific mutations can drive cancer onset and progression. The mutational profile of genes potentially involved in carcinogenesis is thus commonly studied in tumor samples. There are a few reports of mutations affecting Semaphorin or Semaphorin-receptor genes, however they have not been convincingly linked to tumor onset or progression until now. Point mutations in these genes could perturb dimerization, ligand-receptor binding or signal transduction pathways. Mutations could also generate truncated forms of the Plexins, potentially acting as dominant negative or constitutive active molecules.
Interestingly, the expression of Semaphorins seems to be often regulated in cancers. For example, Semaphorin 3B and Semaphorin 3F are considered putative tumor suppressor genes since the chromosomal region 3p21.3 in which they are located is frequently deleted in lung tumors and undergoes promoter silencing by hyper-methylation, leading to reduced expression of these genes.65–70 Moreover it has been reported that the expression of Sema3B and Sema3F is under control of p53 tumor suppressor.53,71 In contrast, Sema3C, Sema3E and Sema5C have been found upregulated in tumors and their expression can promote cancer progression in experimental models.72–78 Intriguingly, it was reported that the developmental expression of Semaphorin 3A is under control of hypoxia-driven factor HIF1α,79 a mechanism that is also often in place during tumor growth and tumor angiogenesis. In fact, although Sema3A is known to inhibit angiogenesis, it is expressed in several cancer cells and it may regulate the anti-tumor immune response;80 therefore, its functional role in cancer progression deserves further investigation in vivo.
Expression of PlexinD1, which is downregulated after embryo development, has been specifically reported in tumor cells and in tumor endothelial cells.81 However the functional role of this finding has not been established. On the other hand, it has been recently reported that PlexinB1 expression is lost in a subset of breast carcinoma characterized by poor prognosis, high proliferative rate and hormone-dependence.82 Neuropilin-1 expression is frequently elevated in tumor cells and correlated with cancer progression; this effect is putatively explained by an ability to promote VEGF signalling in trans in adjacent endothelial cells.58,83 Neuropilin-2 is upregulated in a subset of cancer cells, especially of neural crest origin.84–86 In bladder cancer, Nrp2 expression has been correlated with advanced stage tumors,87 while in gastrointestinal tumors loss of its expression seems to correlate with progression;88 thereby the role of Nrp2 in controlling tumor proliferation remains controversial.
Tumor micro-environment plays an important role in cancer progression. This depends on the recruitment of endothelial cells, leucocytes, fibroblasts and additional stromal cells, and on the growth factors, cytokines and proteases they release. In addition, the extracellular matrix surrounding the tumor regulates cell migration and is a reservoir of growth factors in inactive form. Several members of the Semaphorin family regulate endothelial cell migration and angiogenesis: e.g., Semaphorin 3A, 3F, 3E, 4A and 6A can inhibit angiogenesis.18,22,89–95 Moreover, certain Semaphorins may compete with VEGFs for the binding site on Neuropilins.14,96–97 On the other hand, Semaphorin 4D is a pro-angiogenic factor released by human cancer cells (via MMP-mediated cleavage) and its activity has been shown to mediate tumor growth.98–100 Different leucocytes are recruited to tumor sites via cytokines secretion, and while some of them participate in the anti-tumor immune response, others appear to be responsible for promoting tumor progression. For instance, tumor-associated macrophages (TAMs) are well known to regulate cancer cell invasion and angiogenesis,101 as well as metastatic dissemination. In vivo studies have further shown that TAMs localize preferentially in the proximity of tumor vessels where they can affect permeability and promote tumor metastasis.102 Notably, Semaphorin 4D and Semaphorin 7A have been reported to regulate monocytes migration in vitro.103–106 However, it remains to be seen if semaphorin signals can regulate the recruitment of tumor associated macrophages affecting tumor progression.
Future Perspectives
Plexins and Neuropilins are well known semaphorin receptors; however the molecular mechanisms mediating multifaceted semaphorin functions require further elucidation. For instance, several signal transducers for semaphorins have been identified in experimental studies, and yet the functional relevance in vivo of these multiple pathways, in different tissues and tumor types, remains largely unknown. Future studies, e.g., by proteomic approaches, could lead to the identification of specific molecules associated with Semaphorin receptors on the surface of different cell populations. Moreover, loss-of-function screens could reveal the signal transducers implicated in specific semaphorin functions.
In recent years, several reports have underlined the importance of a minor fraction of the cells forming a tumor mass that is actually endowed with tumor-initiating and tumor-maintaining ability (often indicated as “cancer stem cells”; reviewed in ref. 107). Moreover, cancer-initiating cells may be the only ones which can effectively produce metastatic dissemination. This finding is particularly relevant to medicine, since novel targeted therapies should then aim at hitting this specific cell population in the tumor. However, the identity of these cancer-initiating cells remains elusive, and their behaviour seems to be under control of the tumor microenvironment.108 For instance, it was shown that ephrin-B1 acts as regulatory signal restraining normal stem cells, but not cancer cells, into the intestinal crypt niche.109 Thus, it appears of great importance to identify effective regulatory signals for cancer stem cells. Semaphorins and Plexins may be intriguing candidates for this function, since they are expressed in developmental and tumor tissues, and are known to regulate cell-cell adhesion/dissociation, as well as cell motility and cell differentiation. Future studies will reveal whether there is a role for these signals in the function of normal and neoplastic stem cells.
Table 1.
Semaphorins and semaphorin receptors in cancer
| Receptors Known | Reported Functions Potentially Relevant in Cancer | |
| Sema3A | Nrp1 (+ Plexins) | Inhibits angiogenesis.22,89 Inhibits breast carcinoma cell migration.18 |
| Regulates immune response.80 | ||
| Loss of expression (and loss of auto-inhibitory loops) in mesothelioma and multiple myeloma.51,89 | ||
| Sema3B | Nrp1 and Nrp2 (+ Plexins) | Putative tumor suppressor in different tumor types.65,66,68,110 |
| Inhibits growth and induces apoptosis in tumor cells.50,67,70 | ||
| Sema3C | Nrp1 and Nrp2 (+ Plexins) | Activates Integrin-mediated adhesion, migration and proliferation in endothelial and carcinoma cells.111,112 High expression correlates with metastasis from lung cancer.74 |
| Sema3E | PlexinD1 (Neuropilins ) ) |
Expression associated with the metastatic process.75,76 |
| Repels endothelial cells in development.92 | ||
| Sema3F | Nrp2 (+ Plexins) | Acts as a tumor suppressor gene in experimental models.52–54 |
| Inhibits tumor angiogenesis, lymphangiogenesis, and metastatic progression of melanoma cells.94 | ||
| Sema4A | Tim-2, PlexinD1 | Activates T-cell-mediated immunity via Tim-2.6 Suppresses angiogenesis via Plexin-D1.95 |
| Sema4B | unknown | Interacts with CLCP1, a protein with similarity to neuropilins, overexpressed in metastatic cells derived from lung cancer.113 |
| Sema4D | PlexinB1 (PlexinB2, CD72) | Mediates endothelial cell migration and tumor induced angiogenesis.98–100 |
| Regulates monocytes migration and differentiation.105 | ||
| Promote leukaemia cells growth and survival.55,56 | ||
| It is released during platelet aggregation.114 | ||
| Can trigger the activation of Met oncogene and lead to the invasive growth programme.34 | ||
| PlexinB1 is down regulated in mammary carcinomas with poor prognosis.115 | ||
| Sema5A | PlexinB3 (Proteoglycans) | PlexinB3 can form a complex with Met oncogene and mediate its activation.116 |
| Sema5A may induce antagonistic responses (attraction/repulsion).117 | ||
| Sema5C | unknown | Required for metastatic progression in a fly model of tumorigenesis.78 |
| Sema6A | PlexinA4 | The extracellular domain can be used to inhibit tumor angiogenesis.118 |
| Sema6B | PlexinA4 | Its expression is downregulated by antitumor agents in glioblastoma and mammary carcinoma cells.119,120 |
| Sema6D | PlexinA1 | It can elicit the activation of VEGF-R2 associated in complex with PlexinA1 and trigger invasive growth response in heart development via reverse signalling mediated by its cytoplasmic domain.36,38 |
| Mediates differentiation of dendritic cells and osteoclasts.63 | ||
| Sema7A | PlexinC1, Integrin-β1, others
|
It induces FAK and MAPK activation, via Integrin-β1 engagement.4 |
| It regulates cells of the immune response.121,122 |
Most studied vertebrate semaphorins, their known receptors and functional activities potentially relevant in cancer.
Acknowledgements
The authors wish to thank Davide Barberis, Asha Balakrishnan and all Tamagnone lab members for advice and suggestions, and the Italian Association for Cancer Research (AIRC) for supporting ongoing research in the lab.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/4570
References
- 1.Tamagnone L, Comoglio PM. To move or not to move? Semaphorin signalling in cell migration. EMBO Rep. 2004;5:356–361. doi: 10.1038/sj.embor.7400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- 3.Tamagnone L, Comoglio PM. Signalling by semaphorin receptors: Cell guidance and beyond. Trends Cell Biol. 2000;10:377–383. doi: 10.1016/s0962-8924(00)01816-x. [DOI] [PubMed] [Google Scholar]
- 4.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 5.Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, Hirata H, Iwahori K, Uchida J, Yasui T, Matsumoto M, Yoshida K, Yakura H, Pan C, Parnes JR, Kikutani H. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: A novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 6.Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, Kikutani H. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419:629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- 7.Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 8.Vikis HG, Li W, He Z, Guan KL. The semaphorin receptor plexin-B1 specifically interacts with active Rac in a ligand-dependent manner. Proc Natl Acad Sci USA. 2000;97:12457–12462. doi: 10.1073/pnas.220421797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanata SM, Hovatta I, Rohm B, Puschel AW. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J Neurosci. 2002;22:471–477. doi: 10.1523/JNEUROSCI.22-02-00471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Li M, Chai H, Yang H, Fisher WE, Yao Q. Roles of neuropilins in neuronal development, angiogenesis, and cancers. World J Surg. 2005;29:271–275. doi: 10.1007/s00268-004-7818-1. [DOI] [PubMed] [Google Scholar]
- 12.Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, Mandron M, Herault JP, Neufeld G, Savi P, Herbert JM, Bono F. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–1250. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- 13.Karpanen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, Tamagnone L, Alitalo K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 2006;20:1462–1472. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 15.Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 16.Miao HQ, Klagsbrun M. Neuropilin is a mediator of angiogenesis. Cancer Metastasis Rev. 2000;19:29–37. doi: 10.1023/a:1026579711033. [DOI] [PubMed] [Google Scholar]
- 17.Nasarre P, Constantin B, Rouhaud L, Harnois T, Raymond G, Drabkin HA, Bourmeyster N, Roche J. Semaphorin SEMA3F and VEGF have opposing effects on cell attachment and spreading. Neoplasia. 2003;5:83–92. doi: 10.1016/s1476-5586(03)80020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–5233. [PubMed] [Google Scholar]
- 19.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le CJ, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vieira JM, Schwarz Q, Ruhrberg C. Selective requirements for NRP1 ligands during neurovascular patterning. Development. 2007;134:1833–1843. doi: 10.1242/dev.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Puschel AW, Bussolino F. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Zeng H, Wang P, Soker S, Mukhopadhyay D. Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration. J Biol Chem. 2003;278:48848–48860. doi: 10.1074/jbc.M310047200. [DOI] [PubMed] [Google Scholar]
- 24.Cai H, Reed RR. Cloning and characterization of neuropilin-1-interacting protein: A PSD- 95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci. 1999;19:6519–6527. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang LH, Kalb RG, Strittmatter SM. A PDZ protein regulates the distribution of the transmembrane semaphorin, M-SemF. J Biol Chem. 1999;274:14137–14146. doi: 10.1074/jbc.274.20.14137. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [published erratum appears in Neuron 1997 Sep;19(3):559] [DOI] [PubMed] [Google Scholar]
- 27.Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron. 1998;21:1283–1290. doi: 10.1016/s0896-6273(00)80648-0. [DOI] [PubMed] [Google Scholar]
- 28.Cackowski FC, Xu L, Hu B, Cheng SY. Identification of two novel alternatively spliced Neuropilin-1 isoforms. Genomics. 2004;84:82–94. doi: 10.1016/j.ygeno.2004.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukasawa M, Korc M. Vascular endothelial growth factor-trap suppresses tumorigenicity of multiple pancreatic cancer cell lines. Clin Cancer Res. 2004;10:3327–3332. doi: 10.1158/1078-0432.CCR-03-0820. [DOI] [PubMed] [Google Scholar]
- 30.Mamluk R, Klagsbrun M, Detmar M, Bielenberg DR. Soluble neuropilin targeted to the skin inhibits vascular permeability. Angiogenesis. 2005;8:217–227. doi: 10.1007/s10456-005-9009-6. [DOI] [PubMed] [Google Scholar]
- 31.Schuch G, Machluf M, Bartsch G, Jr, Nomi M, Richard H, Atala A, Soker S. In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood. 2002;100:4622–4628. doi: 10.1182/blood.V100.13.4622. [DOI] [PubMed] [Google Scholar]
- 32.Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, Klagsbrun M. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc Natl Acad Sci USA. 2000;97:2573–2578. doi: 10.1073/pnas.040337597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swiercz JM, Kuner R, Offermanns S. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol. 2004;165:869–880. doi: 10.1083/jcb.200312094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- 35.Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23:5131–5137. doi: 10.1038/sj.onc.1207650. [DOI] [PubMed] [Google Scholar]
- 36.Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legler DF, Doucey MA, Schneider P, Chapatte L, Bender FC, Bron C. Differential insertion of GPI-anchored GFPs into lipid rafts of live cells. FASEB J. 2005;19:73–75. doi: 10.1096/fj.03-1338fje. [DOI] [PubMed] [Google Scholar]
- 38.Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Yabuki M, Harada K, Hori M, Kikutani H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signaling. Nat Cell Biol. 2004;6:1204–1211. doi: 10.1038/ncb1193. [DOI] [PubMed] [Google Scholar]
- 39.Sahai E, Marshall CJ. Differing modes of tumor cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 40.Torka R, Thuma F, Herzog V, Kirfel G. ROCK signaling mediates the adoption of different modes of migration and invasion in human mammary epithelial tumor cells. Exp Cell Res. 2006;312:3857–3871. doi: 10.1016/j.yexcr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 41.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 43.Oinuma I, Katoh H, Negishi M. Semaphorin 4D/Plexin-B1-mediated R-Ras GAP activity inhibits cell migration by regulating β1 integrin activity. J Cell Biol. 2006;173:601–603. doi: 10.1083/jcb.200508204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barberis D, Artigiani S, Casazza A, Corso S, Giordano S, Love CA, Jones EY, Comoglio PM, Tamagnone L. Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J. 2004;18:592–594. doi: 10.1096/fj.03-0957fje. [DOI] [PubMed] [Google Scholar]
- 45.Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, Comoglio PM, Tamagnone L. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci. 2005;118:4689–4700. doi: 10.1242/jcs.02590. [DOI] [PubMed] [Google Scholar]
- 46.Hegerfeldt Y, Tusch M, Brocker EB, Friedl P. Collective cell movement in primary melanoma explants: Plasticity of cell-cell interaction, β1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–2130. [PubMed] [Google Scholar]
- 47.Niggemann B, Drell TL, Joseph J, Weidt C, Lang K, Zaenker KS, Entschladen F. Tumor cell locomotion: Differential dynamics of spontaneous and induced migration in a 3D collagen matrix. Exp Cell Res. 2004;298:178–187. doi: 10.1016/j.yexcr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Banu N, Teichman J, Dunlap-Brown M, Villegas G, Tufro A. Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J. 2006;20:2150–2152. doi: 10.1096/fj.05-5698fje. [DOI] [PubMed] [Google Scholar]
- 49.Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int J Oncol. 2007;30:1231–1238. [PubMed] [Google Scholar]
- 50.Castro-Rivera E, Ran S, Thorpe P, Minna JD. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci USA. 2004;101:11432–11437. doi: 10.1073/pnas.0403969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catalano A, Caprari P, Rodilossi S, Betta P, Castellucci M, Casazza A, Tamagnone L, Procopio A. Cross-talk between vascular endothelial growth factor and semaphorin-3A pathway in the regulation of normal and malignant mesothelial cell proliferation. FASEB J. 2004;18:358–360. doi: 10.1096/fj.03-0513fje. [DOI] [PubMed] [Google Scholar]
- 52.Chabbert-de PI, Buffard V, Leroy K, Bagot M, Bensussan A, Wolkenstein P, Marie-Cardine A. Antiproliferative effect of semaphorin 3F on human melanoma cell lines. J Invest Dermatol. 2006;126:2343–2345. doi: 10.1038/sj.jid.5700382. [DOI] [PubMed] [Google Scholar]
- 53.Futamura M, Kamino H, Miyamoto Y, Kitamura N, Nakamura Y, Ohnishi S, Masuda Y, Arakawa H. Possible role of semaphorin 3F, a candidate tumor suppressor gene at 3p21.3, in p53-regulated tumor angiogenesis suppression. Cancer Res. 2007;67:1451–1460. doi: 10.1158/0008-5472.CAN-06-2485. [DOI] [PubMed] [Google Scholar]
- 54.Xiang R, Davalos AR, Hensel CH, Zhou XJ, Tse C, Naylor SL. Semaphorin 3F gene from human 3p21.3 suppresses tumor formation in nude mice. Cancer Res. 2002;62:2637–2643. [PubMed] [Google Scholar]
- 55.Granziero L, Circosta P, Scielzo C, Frisaldi E, Stella S, Geuna M, Giordano S, Ghia P, Caligaris-Cappio F. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood. 2003;101:1962–1969. doi: 10.1182/blood-2002-05-1339. [DOI] [PubMed] [Google Scholar]
- 56.Deaglio S, Vaisitti T, Bergui L, Bonello L, Horenstein AL, Tamagnone L, Boumsell L, Malavasi F. CD38 and CD100 lead a network of surface receptors relaying positive signals for B-CLL growth and survival. Blood. 2005;105:3042–3050. doi: 10.1182/blood-2004-10-3873. [DOI] [PubMed] [Google Scholar]
- 57.Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]
- 58.Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- 59.Marcus K, Johnson M, Adam RM, O'Reilly MS, Donovan M, Atala A, Freeman MR, Soker S. Tumor cell-associated neuropilin-1 and vascular endothelial growth factor expression as determinants of tumor growth in neuroblastoma. Neuropathology. 2005;25:178–187. doi: 10.1111/j.1440-1789.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 60.Baba T, Kariya M, Higuchi T, Mandai M, Matsumura N, Kondoh E, Miyanishi M, Fukuhara K, Takakura K, Fujii S. Neuropilin-1 promotes unlimited growth of ovarian cancer by evading contact inhibition. Gynecol Oncol. 2007;105:703–711. doi: 10.1016/j.ygyno.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Ochiumi T, Kitadai Y, Tanaka S, Akagi M, Yoshihara M, Chayama K. Neuropilin-1 is involved in regulation of apoptosis and migration of human colon cancer. Int J Oncol. 2006;29:105–116. [PubMed] [Google Scholar]
- 62.Hu B, Guo P, Bar-Joseph I, Imanishi Y, Jarzynka MJ, Bogler O, Mikkelsen T, Hirose T, Nishikawa R, Cheng SY. Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene. 2007 doi: 10.1038/sj.onc.1210348. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K, Ishii M, Terai K, Moriya M, Nakatsuji Y, Sakoda S, Sato S, Akira S, Takeda K, Inui M, Takai T, Ikawa M, Okabe M, Kumanogoh A, Kikutani H. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- 64.Kikutani H, Suzuki K, Kumanogoh A. Immune semaphorins: Increasing members and their diverse roles. Adv Immunol. 2007;93:121–143. doi: 10.1016/S0065-2776(06)93003-X. [DOI] [PubMed] [Google Scholar]
- 65.Ito M, Ito G, Kondo M, Uchiyama M, Fukui T, Mori S, Yoshioka H, Ueda Y, Shimokata K, Sekido Y. Frequent inactivation of RASSF1A, BLU, and SEMA3B on 3p21.3 by promoter hypermethylation and allele loss in non-small cell lung cancer. Cancer Lett. 2005;225:131–139. doi: 10.1016/j.canlet.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 66.Roche J, Boldog F, Robinson M, Robinson L, Varella-Garcia M, Swanton M, Waggoner B, Fishel R, Franklin W, Gemmill R, Drabkin H. Distinct 3p21.3 deletions in lung cancer and identification of a new human semaphorin. Oncogene. 1996;12:1289–1297. [PubMed] [Google Scholar]
- 67.Tomizawa Y, Sekido Y, Kondo M, Gao B, Yokota J, Roche J, Drabkin H, Lerman MI, Gazdar AF, Minna JD. Inhibition of lung cancer cell growth and induction of apoptosis after reexpression of 3p21.3 candidate tumor suppressor gene SEMA3B. Proc Natl Acad Sci USA. 2001;98:13954–13959. doi: 10.1073/pnas.231490898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuroki T, Trapasso F, Yendamuri S, Matsuyama A, Alder H, Williams NN, Kaiser LR, Croce CM. Allelic loss on chromosome 3p21.3 and promoter hypermethylation of semaphorin 3B in non-small cell lung cancer. Cancer Res. 2003;63:3352–3355. [PubMed] [Google Scholar]
- 69.Xiang RH, Hensel CH, Garcia DK, Carlson HC, Kok K, Daly MC, Kerbacher K, van den Berg A, Veldhuis P, Buys CH, Naylor SL. Isolation of the human semaphorin III/F gene (SEMA3F) at chromosome 3p21, a region deleted in lung cancer. Genomics. 1996;32:39–48. doi: 10.1006/geno.1996.0074. [DOI] [PubMed] [Google Scholar]
- 70.Tse C, Xiang RH, Bracht T, Naylor SL. Human Semaphorin 3B (SEMA3B) located at chromosome 3p21.3 suppresses tumor formation in an adenocarcinoma cell line. Cancer Res. 2002;62:542–546. [PubMed] [Google Scholar]
- 71.Ochi K, Mori T, Toyama Y, Nakamura Y, Arakawa H. Identification of semaphorin3B as a direct target of p53. Neoplasia. 2002;4:82–87. doi: 10.1038/sj.neo.7900211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int J Oncol. 2007;30:1231–1238. [PubMed] [Google Scholar]
- 73.Yamada T, Endo R, Gotoh M, Hirohashi S. Identification of semaphorin E as a non-MDR drug resistance gene of human cancers. Proc Natl Acad Sci USA. 1997;94:14713–14718. doi: 10.1073/pnas.94.26.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin-Satue M, Blanco J. Identification of semaphorin E gene expression in metastatic human lung adenocarcinoma cells by mRNA differential display. J Surg Oncol. 1999;72:18–23. doi: 10.1002/(sici)1096-9098(199909)72:1<18::aid-jso5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 75.Christensen C, Ambartsumian N, Gilestro G, Thomsen B, Comoglio P, Tamagnone L, Guldberg P, Lukanidin E. Proteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasis. Cancer Res. 2005;65:6167–6177. doi: 10.1158/0008-5472.CAN-04-4309. [DOI] [PubMed] [Google Scholar]
- 76.Christensen CR, Klingelhofer J, Tarabykina S, Hulgaard EF, Kramerov D, Lukanidin E. Transcription of a novel mouse semaphorin gene, M-semaH, correlates with the metastatic ability of mouse tumor cell lines. Cancer Res. 1998;58:1238–1244. [PubMed] [Google Scholar]
- 77.Rieger J, Wick W, Weller M. Human malignant glioma cells express semaphorins and their receptors, neuropilins and plexins. Glia. 2003;42:379–389. doi: 10.1002/glia.10210. [DOI] [PubMed] [Google Scholar]
- 78.Woodhouse EC, Fisher A, Bandle RW, Bryant-Greenwood B, Charboneau L, Petricoin EF, IIIrd, Liotta LA. Drosophila screening model for metastasis: Semaphorin 5c is required for l(2)gl cancer phenotype. Proc Natl Acad Sci USA. 2003;100:11463–11468. doi: 10.1073/pnas.2031202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Compernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, Collen D, Carmeliet P. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha. Cardiovasc Res. 2003;60:569–579. doi: 10.1016/j.cardiores.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Catalano A, Caprari P, Moretti S, Faronato M, Tamagnone L, Procopio A. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood. 2006;107:3321–3329. doi: 10.1182/blood-2005-06-2445. [DOI] [PubMed] [Google Scholar]
- 81.Roodink I, Raats J, Van Der ZB, Verrijp K, Kusters B, Van Bokhoven H, Linkels M, de Waal RM, Leenders WP. Plexin D1 expression is induced on tumor vasculature and tumor cells: A novel target for diagnosis and therapy? Cancer Res. 2005;65:8317–8323. doi: 10.1158/0008-5472.CAN-04-4366. [DOI] [PubMed] [Google Scholar]
- 82.Rody A, Holtrich U, Gaetje R, Gehrmann M, Engels K, von MG, Loibl S, allo-Danebrock R, Ruckhaberle E, Metzler D, Ahr A, Solbach C, Karn T, Kaufmann M. Poor outcome in estrogen receptor-positive breast cancers predicted by loss of plexin B1. Clin Cancer Res. 2007;13:1115–1122. doi: 10.1158/1078-0432.CCR-06-2433. [DOI] [PubMed] [Google Scholar]
- 83.Parikh AA, Fan F, Liu WB, Ahmad SA, Stoeltzing O, Reinmuth N, Bielenberg D, Bucana CD, Klagsbrun M, Ellis LM. Neuropilin-1 in human colon cancer: Expression, regulation, and role in induction of angiogenesis. Am J Pathol. 2004;164:2139–2151. doi: 10.1016/S0002-9440(10)63772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chabbert-de PI, Buffard V, Leroy K, Bagot M, Bensussan A, Wolkenstein P, Marie-Cardine A. Antiproliferative effect of semaphorin 3F on human melanoma cell lines. J Invest Dermatol. 2006;126:2343–2345. doi: 10.1038/sj.jid.5700382. [DOI] [PubMed] [Google Scholar]
- 85.Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: Expression, regulation, and function. Exp Cell Res. 2006;312:584–593. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 86.Mariani L, Beaudry C, McDonough WS, Hoelzinger DB, Demuth T, Ross KR, Berens T, Coons SW, Watts G, Trent JM, Wei JS, Giese A, Berens ME. Glioma cell motility is associated with reduced transcription of proapoptotic and proliferation genes: A cDNA microarray analysis. J Neurooncol. 2001;53:161–176. doi: 10.1023/a:1012253317934. [DOI] [PubMed] [Google Scholar]
- 87.Sanchez-Carbayo M, Socci ND, Lozano JJ, Li W, Charytonowicz E, Belbin TJ, Prystowsky MB, Ortiz AR, Childs G, Cordon-Cardo C. Gene discovery in bladder cancer progression using cDNA microarrays. Am J Pathol. 2003;163:505–516. doi: 10.1016/S0002-9440(10)63679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen T, Gluzman-Poltorak Z, Brodzky A, Meytal V, Sabo E, Misselevich I, Hassoun M, Boss JH, Resnick M, Shneyvas D, Eldar S, Neufeld G. Neuroendocrine cells along the digestive tract express neuropilin-2. Biochem Biophys Res Commun. 2001;284:395–403. doi: 10.1006/bbrc.2001.4958. [DOI] [PubMed] [Google Scholar]
- 89.Vacca A, Scavelli C, Serini G, Di PG, Cirulli T, Merchionne F, Ribatti D, Bussolino F, Guidolin D, Piaggio G, Bacigalupo A, Dammacco F. Loss of inhibitory semaphorin 3A (SEMA3A) autocrine loops in bone marrow endothelial cells of patients with multiple myeloma. Blood. 2006;108:1661–1667. doi: 10.1182/blood-2006-04-014563. [DOI] [PubMed] [Google Scholar]
- 90.Kessler O, Shraga-Heled N, Lange T, Gutmann-Raviv N, Sabo E, Baruch L, Machluf M, Neufeld G. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004;64:1008–1015. doi: 10.1158/0008-5472.can-03-3090. [DOI] [PubMed] [Google Scholar]
- 91.Neufeld G. Semaphorins in angiogenesis and cancer. 2005 (Ref Type: Generic) [Google Scholar]
- 92.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 93.Dhanabal M, Wu F, Alvarez E, McQueeney KD, Jeffers M, Macdougall J, Boldog FL, Hackett C, Shenoy S, Khramtsov N, Weiner J, Lichenstein HS, Larochelle WJ. Recombinant semaphorin 6A-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biol Ther. 2005;4:659–668. doi: 10.4161/cbt.4.6.1733. [DOI] [PubMed] [Google Scholar]
- 94.Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest. 2004;114:1260–1271. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, Hori M. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J. 2007;26:1373–1384. doi: 10.1038/sj.emboj.7601589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: Functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85:357–368. doi: 10.1002/jcb.10140. [DOI] [PubMed] [Google Scholar]
- 98.Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem. 2007;282:6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- 99.Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci USA. 2006;103:9017–9022. doi: 10.1073/pnas.0508825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood. 2005;105:4321–4329. doi: 10.1182/blood-2004-07-2885. [DOI] [PubMed] [Google Scholar]
- 101.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: Modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 102.Yamaguchi H, Pixley F, Condeelis J. Invadopodia and podosomes in tumor invasion. Eur J Cell Biol. 2006;85:213–218. doi: 10.1016/j.ejcb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 103.Delaire S, Elhabazi A, Bensussan A, Boumsell L. CD100 is a leukocyte semaphorin. Cell Mol Life Sci. 1998;54:1265–1276. doi: 10.1007/s000180050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Delaire S, Billard C, Tordjman R, Chedotal A, Elhabazi A, Bensussan A, Boumsell L. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J Immunol. 2001;166:4348–4354. doi: 10.4049/jimmunol.166.7.4348. [DOI] [PubMed] [Google Scholar]
- 105.Chabbert-de Ponnat I, Marie-Cardine A, Pasterkamp RJ, Schiavon V, Tamagnone L, Thomasset N, Bensussan A, Boumsell L. Soluble CD100 functions on human monocytes and immature dendritic cells require plexin C1 and plexin B1, respectively. Int Immunol. 2005;17:439–447. doi: 10.1093/intimm/dxh224. [DOI] [PubMed] [Google Scholar]
- 106.Holmes S, Downs AM, Fosberry A, Hayes PD, Michalovich D, Murdoch P, Moores K, Fox J, Deen K, Pettman G, Wattam T, Lewis C. Sema7A is a potent monocyte stimulator. Scand J Immunol. 2002;56:270–275. doi: 10.1046/j.1365-3083.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- 107.Clarke MF, Fuller M. Stem cells and cancer: Two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 108.Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Reprogramming metastatic tumor cells with embryonic microenvironments. Nat Rev Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 109.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de WM, Pawson T, Clevers H. β-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 110.Nair PN, McArdle L, Cornell J, Cohn SL, Stallings RL. High-resolution analysis of 3p deletion in neuroblastoma and differential methylation of the SEMA3B tumor suppressor gene. Cancer Genet Cytogenet. 2007;174:100–110. doi: 10.1016/j.cancergencyto.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 111.Banu N, Teichman J, Dunlap-Brown M, Villegas G, Tufro A. Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J. 2006;20:2150–2152. doi: 10.1096/fj.05-5698fje. [DOI] [PubMed] [Google Scholar]
- 112.Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int J Oncol. 2007;30:1231–1238. [PubMed] [Google Scholar]
- 113.Nagai H, Sugito N, Matsubara H, Tatematsu Y, Hida T, Sekido Y, Nagino M, Nimura Y, Takahashi T, Osada H. CLCP1 interacts with semaphorin 4B and regulates motility of lung cancer cells. Oncogene. 2007;26:4025–4031. doi: 10.1038/sj.onc.1210183. [DOI] [PubMed] [Google Scholar]
- 114.Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, Tamagnone L, Wagner DD, Milla ME, Brass LF. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci USA. 2007;104:1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rody A, Holtrich U, Gaetje R, Gehrmann M, Engels K, von MG, Loibl S, allo-Danebrock R, Ruckhaberle E, Metzler D, Ahr A, Solbach C, Karn T, Kaufmann M. Poor outcome in estrogen receptor-positive breast cancers predicted by loss of plexin B1. Clin Cancer Res. 2007;13:1115–1122. doi: 10.1158/1078-0432.CCR-06-2433. [DOI] [PubMed] [Google Scholar]
- 116.Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 2004;5:710–714. doi: 10.1038/sj.embor.7400189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 118.Dhanabal M, Wu F, Alvarez E, McQueeney KD, Jeffers M, Macdougall J, Boldog FL, Hackett C, Shenoy S, Khramtsov N, Weiner J, Lichenstein HS, Larochelle WJ. Recombinant semaphorin 6A-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biol Ther. 2005;4:659–668. doi: 10.4161/cbt.4.6.1733. [DOI] [PubMed] [Google Scholar]
- 119.Correa RG, Sasahara RM, Bengtson MH, Katayama ML, Salim AC, Brentani MM, Sogayar MC, de Souza SJ, Simpson AJ. Human semaphorin 6B [(HSA)SEMA6B], a novel human class 6 semaphorin gene: Alternative splicing and all-trans-retinoic acid-dependent down-regulation in glioblastoma cell lines. Genomics. 2001;73:343–348. doi: 10.1006/geno.2001.6525. [DOI] [PubMed] [Google Scholar]
- 120.Collet P, Domenjoud L, Devignes MD, Murad H, Schohn H, Dauca M. The human semaphorin 6B gene is down regulated by PPARs. Genomics. 2004;83:1141–1150. doi: 10.1016/j.ygeno.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 121.Walzer T, Galibert L, De Smedt T. Dendritic cell function in mice lacking Plexin C1. Int Immunol. 2005;17:943–950. doi: 10.1093/intimm/dxh274. [DOI] [PubMed] [Google Scholar]
- 122.Walzer T, Galibert L, De Smedt T. Poxvirus semaphorin A39R inhibits phagocytosis by dendritic cells and neutrophils. Eur J Immunol. 2005;35:391–398. doi: 10.1002/eji.200425669. [DOI] [PubMed] [Google Scholar]


