Abstract
The term polarity refers to the differential distribution of the macromolecular elements of a cell, resulting in its asymmetry in function, shape and/or content. Polarity is a fundamental property of all metazoan cells in at least some stages, and is pivotal to processes such as epithelial differentiation (apical/basal polarity), coordinated cell activity within the plane of a tissue (planar cell polarity), asymmetric cell division, and cell migration. In the last case, an apparently symmetric cell responds to directional cues provided by chemoattractants, creating a polarity axis that runs from the cell anterior, or leading edge, in which actin polymerization takes place, to the cell posterior (termed uropod in leukocytes), in which acto-myosin contraction occurs. Here we will review some of the molecular mechanisms through which chemoattractants break cell symmetry to trigger directed migration, focusing on cells of the immune system. We briefly highlight some common or apparently contradictory pathways reported as important for polarity in other cells, as this suggests conserved or cell type-specific mechanisms in eukaryotic cell chemotaxis.
Key Words: chemotaxis, polarization, lipid rafts, signaling, cytoskeleton
Introduction
To perform a specific function at a given time, a cell must change its position within the organism. This process involves activation of a program that enables the cell to move. Migration is a key event in physiological processes such as embryo implantation and development, tissue repair, angiogenesis and the immune response. Deregulation of the migration program is also an important component in several pathologies, including chronic inflammation, autoimmunity and tumor metastasis. The molecular mechanisms that initiate and regulate cell migration in physiological and pathological situations are similar, although not identical.1,2 Understanding these systems would therefore enable not only comprehension of distinct physiological processes, but would also allow intervention in diseases in which cell migration has a role.
Two main processes regulate migration in most eukaryotic cells: chemotaxis and chemokinesis. Chemotaxis refers to directed migration of cells towards a gradient of a soluble chemoattractant or an extracellular matrix (ECM) component; chemokinesis is an increase in random, undirected cell motility. Whether these processes are governed by the same molecular mechanisms is not known, but all follow three basic principles. First, cells must develop morphological and functional asymmetry to migrate; in other words, migrating cells must become polarized. This polarization segregates two cell compartments with specific properties, composition and functions: the leading edge at the front and the uropod at the rear. Second, cell migration is a cyclic process, involving the extension of protrusions (pseudopodia, lamellipodia and filopodia) at the cell front and retraction at the cell back. Finally, the ability to move requires generation of traction forces, which are balanced by cell adhesion to the extracellular matrix. On overly sticky surfaces, cells flatten but cannot crawl, whereas on insufficiently sticky surfaces, cells cannot generate traction forces to move forward.
This review will focus on the spatial and functional polarization of immune cells engaged in chemotaxis. Our present knowledge of how cells achieve polarization in response to chemoattractants is the sum of data derived from studies of different cell types, modes of migration, and environments. Although some of these results appear contradictory, other pieces of the puzzle are observed in many of the systems analyzed, indicating that they are solid elements of the cell polarity program. We will center on these components, as they highlight the basic molecular mechanisms involved in polarity among different cell types.
Chemotactic Signals
Chemoattractants are the spatial signals that initiate and maintain cell polarization during chemotaxis. There are two large chemoattractant groups for eukaryotic cells, those that bind to seven-transmembrane receptors coupled to heterotrimeric G proteins (GPCR), and those that act through tyrosine kinase receptors. In immune cells, the chemokines are among the most prominent chemotactic molecules that act through GPCR; they are a superfamily of more than 50 members involved principally in mobilization of immune system cells.3 The second group of chemoattractants consists mainly of growth factors that act through receptors with intrinsic tyrosine kinase activity. Most of these growth factors induce chemotaxis in epithelial and mesenchymal cells, to which chemokines are poor chemoattractants; importantly, growth factors also induce chemotaxis of tumor cells, in some cases increasing their metastatic potential.4–7
Signaling Pathways in Chemoattractant-Induced Polarity
During chemotaxis, a cell must determine the general direction of the signal source and orient itself accordingly. This is possible since chemotaxing cells are extremely sensitive to small differences in chemoattractant concentrations. Eukaryotic cells are able to detect differences in chemoattractant concentrations across the cell length (spatial sensing), and simultaneously sense time-dependent changes in signal concentration during movement (temporal sensing). Both spatial and temporal sensing are regulated by the interplay of various signaling pathways and other cellular events, presumably connected to the actin polymerization machinery, which is the major force that drives polarity. In neutrophils and lymphocytes, this polarity is very persistent; a 180° change in gradient direction usually leads cells to make U-turns.8 This contrasts with Dictyostelium cells, in which polarity is a fairly transient state, and a cell usually develops a new leading edge when the gradient source changes.
The establishment and maintenance of persistent cell polarization in shallow chemoattractant gradients appear to be mediated by a set of feedback loops involving phosphatidylinositol 3-kinases (PI3K), the Rho family of small GTPases, integrins, and PDZ-containing proteins, as well as microtubule and vesicular transport and plasma membrane composition. In the following sections, we will analyze the molecular machinery that underlies the polarity program induced by GPCR agonists.
Heterotrimeric G proteins.
Chemoattractant binding to a GPCR triggers dissociation of the Gαβγ trimer, to generate free Gα and the dimer Gβγ, as well as GDP/GTP interchange in the Gα subunit. Both Gα and Gβγ control the activity of effector enzymes and ionic channels.9 Although GPCR can associate to different subclasses of trimeric G proteins, most (if not all) receptors able to induce chemotaxis use pertussis toxin (PTx)-sensitive inhibitory G proteins (Gi).3 Evidence suggests that the Gαi subunit, although necessary, is not sufficient to induce polarity and chemotaxis.10 It was proposed that chemokine receptors trigger activation of Janus kinases (Jak),11 which might be an important step in Gαβγ trimer-mediated signaling. Jak activation, probably mediated by receptor dimerization, induces chemokine receptor phosphorylation in tyrosine residues. This exposes residues critical for Gi protein binding.3 Lack of JAK signaling is reported to promote serious defects in chemokine-induced cell chemotaxis.12–14 In another study, however, chemokine-induced Ca2+ flux (a classical Gi-dependent signaling event) and chemotaxis were unaffected in Jak3-deficient lymphocytes or cells transfected with siRNA for Jak2.15 The reasons for these discrepancies require further investigation.
Rho small GTPases.
Cell migration depends largely on the dynamic remodeling of actin cytoskeletal elements.16 Remodeling is controlled through a plethora of effectors activated by the Rho family of small GTPases. These proteins cycle between active (GTP-bound) and inactive (GDP-bound) states through the activity of three groups of proteins: GEF (guanine nucleotide exchange factors), which trigger the Rho-GTP-bound state, GAP (GTPase-activating proteins), which increase the Rho-GDP-bound state, and GDI (guanosine dissociation inhibitors), whose binding prevents anchorage of the Rho GTPases to cell membranes.17,18
There are more than 20 members of this GTPase family in mammals, which can be divided into seven subfamilies: Rho, Rac, Cdc42, RhoD, RhoG, RhoE and TC10. The different Rho GTPases have specific roles in F-actin remodeling. In particular, Rac and Cdc42 are associated with protrusion of the leading edge and directionality of migration. These GTPases control the activity of the Arp2/3 complex at the cell front.19 The Arp2/3 complex constitutes the machinery of actin nucleation and branching by interaction with WASP (Wiskott-Aldrich Syndrome protein) and WAVE proteins (WASP-family verprolin-homologous proteins). Through local actin nucleation, Rac and Cdc42 promote lamellipodium and filopodium formation, respectively.20
Using fluorescent probes, Itoh et al. reported that Cdc42 is most active at the tip of the leading edge of HT1080 cells, and that activity decreases sharply when cells change direction.21 There is evidence that activated Cdc42 is also found at the leading edge of moving leukocytes.22 In addition to Arp2/3 complex regulation, Cdc42 can mediate spatial restriction of lymphocyte lamellipodia by regulating linkage of microtubules (MT) to the cortical cytoskeleton through IQGAP (IQ motif containing GTPase-activating protein 1) and cytoplasmic linker protein-170.23 The MT system is important in establishing persistent polarization; depolymerization of the MT array before stimulation produces the extension of two opposing lateral lamellipodia in neutrophils.24 In addition, the MT system is implicated in mitochondrial polarity in several cell types, a major event in myosin II phosphorylation.25
Although Cdc42 is needed for leading edge formation, this GTPase alone is not sufficient to promote anterior-posterior polarity. Overexpression of a dominant negative Cdc42 mutant hampers macrophage polarization in the direction of the gradient, although these cells can establish a leading edge and a uropod.26 In contrast, Rac inhibition impedes morphologic polarization as well as leading edge accumulation of actin polymers,27 indicating that forward protrusion is probably Rac-mediated.
The GTPase RhoA activates the protein kinase ROCK, which regulates myosin light chain (MLC) phosphorylation, thus increasing F-actin contraction. Conventional myosin II forms a hexamer, composed of two MHC (myosin heavy chains) as well as two pairs of essential, regulatory MLC, which assemble into bipolar filaments with ATPase activity and actin binding capacity.28 Actin-myosin filament assembly stabilizes the actin cytoskeleton and, through ATP-driven translocation of actin filaments, provides the motor activity necessary for efficient cell migration.29 Myosin II activity is regulated by MLC phosphorylation, which can be catalyzed by MLC kinase or negatively regulated by MLC phosphatase. ROCK induces contraction by a mechanism involving MLC phosphatase inactivation and direct MLC phosphorylation.30 Both RhoA and myosin II localize at the sides and rear of chemotactic leukocytes, where they promote cell body contraction and posterior retraction, and simultaneously antagonize Rac to prevent lateral pseudopodium formation.31 In contrast, Rac/Cdc42-induced PAK1 activation at the cell front leads to phosphorylation and inactivation of MLC kinase and MHC II-A, producing a loss in contractility that favors leading edge extension.
The protrusive ability of monocytes is reported to be particularly active when RhoA is inhibited,32 suggesting a RhoA:Rac antagonism that might be critical in establishing front-rear polarity. The antagonism between RhoA and Rac signaling might be pivotal for cell polarity in neurons33 and neutrophils.34 In neutrophils, chemoattractant receptors trigger two divergent signaling pathways initiated by the trimeric Gi and G12/13 proteins, leading to Rac/Cdc42 and RhoA activation, respectively.34
The picture of Rac accumulating at the leading edge and RhoA at the uropod is not so simple, however. Activated Rac has also been detected in the retracting tail of moving neutrophils, using a FRET (fluorescence resonance energy transfer)-based biosensor for Rac activity.35 This result concurs with the inefficiency of uropod retraction in Rac1-deficient neutrophils.36 Both Rac and Cdc42 were recently shown to positively regulate RhoA-myosin II function at the uropod of chemotaxing leukocytes,37,38 although it is not known how these GTPases work in concert between the cell front and rear. Moreover, RhoA biosensors show high RhoA activity levels at the front of randomly migrating fibroblasts,39 in contrast to leukocytes. In these studies, active RhoA levels were greatly attenuated at the cell protrusions when platelet-derived growth factor (PDGF) was used as chemoattractant, suggesting that PDGF-induced Rac activation suppresses RhoA activity at the fibroblast leading edge. In support of this RhoA/Rac antagonism in cells other than leukocytes, RhoA activity can trigger activation of FilGAP, a GAP for Rac, inhibiting Rac function in mesenchymal-like cells; interestingly, ROCK-induced FilGAP activation suppresses leading lamellae formation and promotes retraction.40 These results suggest a requirement for high Rac activity levels at the cell front for protrusion of chemoattractant-stimulated mesenchymal-like cells. This elevated Rac activity might be achieved in part by downmodulation of local RhoA function; however, it is not evident whether RhoA activity is concentrated at the tail of fibroblast-like cells to the same extent as in the leukocyte uropod. Comprehension of RhoA/Rac antagonism in distinct cell types and for different modes of migration will clearly require additional study.
Rap1 (regulator for adhesion and polarization enriched in lymphoid tissues) is another small GTPase that has attracted much attention because of its involvement in several aspects of lymphocyte polarity and migration.41,42 Lymphocytes expressing a constitutively active Rap1 mutant polarize spontaneously and show increased cell migration;43 in contrast, Rap1-deficient T cells have severe polarization defects.44 Rap1-mediated control of cell motility and polarity probably involves regulation of adhesion. Rap1 controls cell adhesion by modulating integrins β1, β2 and β3, in part through the Rap1-binding protein RapL.45,46
Adhesion regulation is necessary to enable cell movement, not only by providing the traction forces required for cell advance, but also through spatial control of the activation of signal transducers important for polarization itself. Indeed, Rac activation is both stimulus- and adhesion-dependent in neutrophils.36 In fibroblasts, integrins recruit Rac to the membrane, as well as restricting Rac activation by displacing Rho-GDI, which blocks effector binding.47 Although chemoattractants may dictate global Rac activation in the cell, integrins would determine the local areas at which Rac binds to effectors; this could explain why chemotaxing cells require integrin interaction with the ECM to establish full polarity.48 Rap1 could position Rac activation by triggering integrin activation, but might also promote Rac signaling indirectly, since Rap1 interacts with the RacGEF Vav2 and Tiam-1.49,50 The fact that the Rapl homologue in yeast, BUD1, participates in polarized bud formation51 suggests that Rapl/RAPL may be a conserved master element in the cell polarization pathway.
Phosphatidylinositol-3 kinases.
One of the first events in chemoattractant signaling is PI3K activation. PI3K are normally heterodimeric proteins consisting of catalytic and regulatory subunits.13,52 Based on these subunits, the PI3K have been grouped in three classes (Table 1), which vary in structure and regulation.53 These kinases catalyze phosphoinositide phosphorylation at the 3′ position of the inositol ring; in vivo, PI3K mainly phosphorylates phosphatidylinositol 4,5 bisphosphate (PIP2) to generate phosphatidylinositol 3,4,5 triphosphate (PIP3). PIP3 generation recruits effector proteins containing pleckstrin homology (PH) domains, which interact specifically with PIP3 or 3′-phosphorylated inositides.
Table 1.
The PI3K family
| Regulatory Subunit | Catalytic Subunit | ||
| CLASS I | Ia | p85α, p85β, p55γ | p110α, p110β, p110δ |
| Ib | p101 | p110γ | |
| CLASS II | ? | PI3KC2α, PI3KC2β | |
| CLASS III | p150 | Vps34p homologue |
The table shows the regulatory and catalytic subunits of the three classes of PI3K. The heterodimeric class Ia PI3K signal downstream of tyrosine kinases and Ras. The p85α regulatory subunit may generate p55α and p50α by alternative splicing. Class Ib PI3K signal downstream of GPCR and Ras. There is little information on the mechanism of activation for class II PI3K, although PI3KC2β has been implicated in lysophosphatidic acid-mediated migration of mesenchymal-like cells. The class III PI3K uses unphosphorylated phosphatidylinositol as a substrate to produce PI3P.
The concept that PI3K is a key player in gradient sensing and cell polarity during chemotaxis is based on experiments using GFP-tagged PH domains as bioprobes to detect the spatial distribution of PI3K products. Studies in different cell types, including Dicytostelium, as well as mammalian fibroblasts and leukocytes, show that PH-containing proteins are recruited selectively to the leading cell edge after exposure to chemoattractant stimuli.54,55 In Dictyostelium, PIP3 is restricted to the leading edge due to the location of PI3K at the cell front and of PTEN (phosphatase and tensin homolog in chromosome 10), the enzyme that dephosphorylates the 3′ position of this lipid, at the rear and sides of the moving cell.56,57 In mammalian cells this model is debated, however; whereas PI3K translocation from cytosol to the leading edge was observed in many cells during chemotaxis, results on uropod localization of PTEN in these cells are contradictory.58–60
PI3Kγ, the only class Ib isoform, is activated by direct Gbγ interaction with the p101 regulatory subunit. Studies involving PI3Kγ overexpression or deficiency suggest a role for this isoform in neutrophil and macrophage migration.61–64 Nonetheless, PI3Kγ deficiency affects T and B lymphocyte polarization and chemotaxis only subtly.65 Accordingly, PTEN deficiency does not affect cell directionality, although its lack usually results in increased cell speed.58,66–68
PIP2-mediated signaling.
Growing evidence shows the function of other lipids in integrating front-rear signaling. One of the most important is PIP2, a direct regulator of many actin-binding and -remodeling proteins, including Rho GTPases.69,70 At the leading cell edge, PIP2 is a substrate shared by PI3K and phospolipase C (PLC). As mentioned above, PIP2 phosphorylation by PI3K generates PIP3, a hallmark of the leading edge in polarized cells. PLC hydrolysis of PIP2 generates inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG), necessary for Ca2+ mobilization and protein kinase C (PKC) activation, respectively.71 PLC activity is necessary for T cell chemotaxis via a Ca2+-independent/DAG-dependent mechanism.72 As we discuss below, DAG-induced PKC activation might be critical for activating well-conserved “polarity cassettes”. PIP2 may also regulate cofilin location at the pseudopodia of carcinoma cells, which is proposed as another guidance system linked to the PLC-PKC pathway.73,74
At the uropod, PIP2 is a major regulator of ERM (ezrin, radixin, moesin) protein activation during leukocyte chemotaxis.75,76 Several adhesion receptors cluster at the uropod, including intercellular adhesion molecules (ICAM), CD43 and CD44.54 This concentration is essential for orchestrating adhesive interactions between leukocytes and the vascular or lymphatic endothelia during diapedesis from blood to tissue and from tissue to lymph nodes. ERM protein interactions with the cytosolic tails of these adhesion receptors might be a mechanism for their uropod polarization. ERM protein activation is a two-step process that requires binding to PIP2 and phosphorylation of C-terminal serine/threonine residues. Several kinases have been implicated in this phosphorylation step, including some PKC isoforms and the RhoA effector ROCK.77 Remarkably, ERM proteins can also act upstream of RhoA by interacting with Rho-GDI, enabling positive feedback between RhoA and ERM proteins.77 This feedback loop may be more complex, since Rac might stimulate ERM dephosphorylation.78 There is thus probably both positive and negative regulation between ERM proteins and Rho GTPases, allowing precise spatio-temporal control during leukocyte chemotaxis. In agreement with this idea, ERM proteins are pivotal in T cell polarity.79
Given the broad range of potential PIP2 targets, compartmentalization of PIP2 inside the cell may be crucial during chemotaxis. Local control of synthesis could be a mechanism for PIP2 compartmentalization. Although PIP2 can be synthesized from PI5P,80 the main biosynthetic pathway is regulated by the so-called type I phosphatidylinositol-4-phosphate 5-kinases (PIP5KI), of which there are three isoforms (α, β and γ);81 little is known, however, about the localization of these isoforms during migration. The PIP5KIα isoform could contribute to localized PIP2 synthesis at the leading edge of migrating fibroblasts by interacting with the LIM protein Ajuba.82 Since Ajuba interaction triggers PIP5KIα activity, and PIP2 is required for Rac activation, Ajuba might be further augmented by Rac1 activity at the cell front.83 Notably, activated Rac1 initiates PIP5KIα translocation to membrane ruffles, suggesting a positive feedback loop involved in cell front protrusion.
PDZ-dontaining protein networks.
Polarity is not restricted to migrating cells, but is also a fundamental property of other cell types such as epithelial cells or neurons. In epithelial cells, polarity is governed by a protein network composed of several functional complexes, including the Scribble, Par, Crumbs and core PCP complexes.84,85 Components of these polarity networks, which are extremely well conserved evolutionarily, were recently implicated in chemokine-induced T cell polarization. The Scribble and Par complexes in particular are needed for directed T lymphocyte migration.86
The Scribble complex comprises three proteins, Scribble, Discs large (Dlg) and Lethal giant larvae (Lgl), all thought to behave as scaffold proteins and regulate protein-protein interactions.87 Scribble effects on polarity might also be mediated by physical interaction with several other proteins, including the Rho GTPase regulatory βPIX-GIT1 complex.88 The Par complex consists of Par3 (known as Bazooka in Drosophila) and Par6, both PDZ-domain-containing scaffold proteins, and aPKCζ, a serine/threonine protein kinase.85 PAR-6 acts in part as a targeting subunit for aPKCζ, to which it binds constitutively. The PAR-6-aPKCζ complex can also bind to and phosphorylate the ubiquitin E3 ligase Smurf1, triggering local RhoA degradation.89 The PAR-6-aPKCζ complex might therefore prevent inappropriate RhoA effects on actin cytoskeleton remodeling, and enable Cdc42 and Rac1 activation to drive rapid filopodial and lamellipodial membrane extension. PAR-6-aPKCζ also binds to and phosphorylates PAR-3,90,91 which interacts with and spatially restricts Tiam-1 activity.92,93 PAR-3 association with LIM kinase (LIMK) could further modulate actin in the area through LIMK regulation of cofilin,92 which may control directionality in carcinoma cells (see above).
Whether Scribble and Par complexes cooperate or antagonize to achieve cell polarity and directed cell migration is a major conundrum. Studies in astrocytes suggest that these two complexes cooperate during migration.94 The Scribble complex might trigger Cdc42 activation through βPIX-GIT1; activated Cdc42 would in turn trigger the Par complex, eliciting Par6-aPKC-dependent signaling in astrocyte migration.94 This contrasts with the classical view, in which Par and Scribble complexes repel each other during epithelial cell polarization.95 This results in asymmetric Par/Scribble distribution across the cell, with the Scribble complex concentrated in the basolateral compartment and the Par complex in the apical section. Spatial segregation and functional antagonism of Par and Scribble complex members are also apparent in polarized T cells during chemotaxis;86 the Scribble complex concentrates at the uropod, whereas the Par complex localizes at the leading edge. The polarity impairment observed in T cells with reduced Scribble levels suggests the functional relevance of spatial segregation of these two pathways, although the mechanisms by which Scribble and Par antagonism controls polarity is not known.
Plasma Membrane domains as organizers of Polarity
Channeling of the information provided by polarity signals, as well as the ability of the cytoskeleton to deform the cell structure depend largely on the physico-chemical properties of the plasma membrane. Given the different functions of the anterior and posterior parts of moving cells, it could be predicted that the leading edge and uropod plasma membranes differ in composition. Indeed, this is seen in epithelial cells, in which the lipid composition of basolateral membranes is distinct from that of the apical membrane. In epithelial cells and neurons, it is proposed that the membrane microdomains termed lipid rafts act as platforms for selective delivery of proteins to specific cell regions, thus reinforcing functional polarity.96,97 Similarly, membrane domains with specialized lipid composition are distributed asymmetrically in several types of moving cells; this may have important consequences in the persistence of cell polarity.98 Since a common feature of these domains is their cholesterol enrichment, we will designate them here as lipid rafts, although this is an oversimplification of the true complexity of plasma membrane domain segregation in migrating cells.99
Raft segregation takes place shortly after chemoattractant stimulation, and depends on chemoattractant receptor signaling and actin cytoskeleton integrity.100 Chemosensory receptors of the chemokine family, such as CXCR4, CCR5, CCR2 and CXCR1, N-formyl peptide receptors, the epidermal growth factor receptor, CD44 and ICAM, among other membrane receptors, are reported to partition in rafts and to be redistributed in migrating cells.101 Moreover, several reports indicate that raft partitioning influences activation and signaling of some of these receptors.102–105 Remarkably, raft-associated receptors redistribute to both the leading edge and the uropod of polarized leukocytes. After chemoattractant-induced polarization, leukocytes segregate two distinct raft subtypes, one to the leading edge and one to the uropod (Fig. 1).106 Leading edge rafts are enriched in ganglioside GM3 and chemosensory receptors, whereas uropod rafts are enriched in ganglioside GM1 and intercellular adhesion receptors. In other cell types, such as endothelial cells, lipid rafts polarize to the cell front during transmigration, but to the rear when these cells migrate in a two-dimensional system.107
Figure 1.
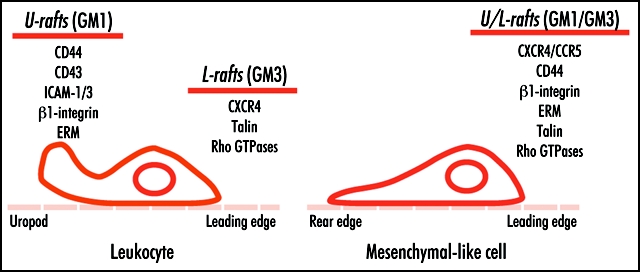
Segregation of lipid rafts in leukocytes and mesenchymal-like cells. The scheme depicts leading edge and posterior markers whose association to lipid rafts has been described. Some uropod markers in leukocytes, such as ERM proteins and the adhesion receptors β1 integrin and CD44, localize at the cell front in mesenchymal cells. This probably reflects the different migratory strategies used by each cell type.
The use of fluorescent proteins has allowed visualization of lipid raft dynamics in leukocytes engaged in chemotaxis. Real-time confocal videomicroscopy studies showed that a lipid raft probe (glycosylphosphatidyl-tagged GFP; GFP-GPI) redistributes to and persists at the leading edge and the uropod in directionally-stimulated lymphocytes, as well as promyelocytic and neutrophil-like cells.108 Similar redistribution was observed using a probe for the inner leaflet of lipid rafts,109 suggesting that inner and outer raft leaflets are coupled during the polarization process. In contrast, a transmembrane non-raft GFP probe (GFP-GT46) showed non-polarized distribution during chemotaxis in these cells.108
Current evidence suggests that rafts are platforms in which efficient interactions take place between activated receptors and signal transduction partners. Double-acylated Gαi subunits concentrate in lipid rafts.110,111 Active chemoattractant receptors and G proteins thus concentrate in a common lipid environment, enabling signaling. Since chemoattractant receptors show preferential affinity for lipid rafts distributed at the leading edge, microdomain redistribution could allow spatial restriction of G protein activation at the cell front. In migrating cells, lipid rafts might increase signaling efficiency, and also restrict and/or organize signaling to specific cell areas. Concurring with this idea, alteration of lipid raft composition impedes functional and morphological polarization of different cell types.100,106 Indeed, lipid rafts can organize activation and/or recruitment of the small GTPases implicated in F-actin remodeling,112,113 and crosslinking of lipid raft components triggers Rho GTPase-dependent actin cytoskeleton rearrangements.114,115
Finally, regulation of membrane elasticity is an emerging new function of specific raft domains in the achievement of cell polarity. Actin cytoskeleton-induced membrane deformation is lower in artificial membranes with low cholesterol content than in membranes with physiological cholesterol levels.116 In endothelial cells, cholesterol depletion results in a significant decrease in membrane deformability and a corresponding increase in the elastic coefficient of the membrane, indicating that cholesterol-depleted cells are stiffer than control cells.117 This is a paradox, as it is well-established that cholesterol addition to artificial phospholipid bilayers increases their rigidity.118 Increased membrane stiffness in cholesterol-depleted cells is reversed by latrunculin A treatment, suggesting that cholesterol can regulate rigidity by altering the properties of submembrane F-actin and/or its membrane association.117 Lipid raft accumulation at the leading edge may thus control local stability of the F-actin network, enabling efficient F-actin-induced membrane protrusions at the cell front. The relationships between cholesterol-enriched membranes and the actin cytoskeleton may nonetheless be bidirectional. Using giant unilamellar vesicles, Liu and Fletcher showed that actin polymerization induces membrane phase separation of initially homogenous vesicles.119 Their results suggest that dynamic, membrane-bound actin networks alone can contribute to membrane organization in polarized cells by controlling when and where lipid rafts form.
Conclusions and Future Directions
A requisite for cell migration is the acquisition of functional and morphological asymmetry. Cell polarity links two basic components of the migration program: motility and directionality. Here we have outlined several molecular mechanisms that, in response to a directional cue, dictate the anterior-posterior asymmetry axis in a cell. The signaling pathways that control front/back polarization involve amplification of PIP3 production at the leading edge, differential activation of Rho family proteins in the cell front and back, PKC activation, protein networks assembled by “polarity proteins” (Scribble and Par complexes), and differential localization of membrane microdomains (lipid rafts).
How the cell senses gradients, and how it processes this information to produce directed motion remain to be worked out. In one attractive model, antagonism between signaling pathways constitutes the major force that creates cell domains involved in protrusion and contraction (Fig. 2). Seminal studies of chemotactic cell migration in Dictyostelium amoebae highlighted the importance of PI3K/PTEN antagonism in these processes. Nonetheless, studies in mammalian cells have not reached a consensus on the importance of local PI3K activity during chemotaxis and have not localized unequivocally PTEN at the posterior edge. There are, moreover, many examples in which PI3K signaling seems to contribute little, if at all, to the polarization program in response to chemoattractants. Neutrophil chemotaxis studies suggest that antagonism between Rac and RhoA GTPases is necessary for cell polarization; the precise mechanisms that control the differential activation of these pathways is nonetheless unclear. The details of how Rac/Cdc42 at the leading edge and RhoA at the uropod work in concert also remain a mystery. There is evidence in T lymphocytes that antagonism between Par and Scribble complexes dictates the establishment of a front/rear polarity axis; nonetheless, Par and Scribble complexes appear to cooperate during migration of other cell types, such as astrocytes. How Par and Scribble complexes activate and coordinate the local and global signaling pathways that regulate cell polarity during chemotaxis is also largely unknown at present. The localization of different types of membrane microdomains at the front and the rear of migrating leukocytes may also be a mechanism that permits or restricts specific signaling involved in leading edge protrusion or uropod contraction. It nonetheless remains to be determined whether the location of these microdomains is the cause or a consequence of establishment of a front/rear polarity axis during migration. Another important question is whether these antagonistic functions are cell type-specific or common to all moving cells.
Figure 2.
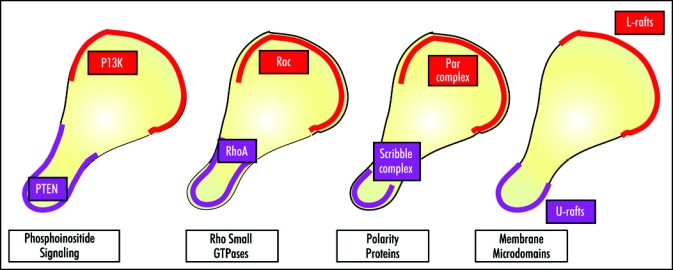
Polarity systems in motile cells. Polarity can be described from the perspective of the antagonistic activity of the PI3K/PTEN system, as described for Dictyostelium orientation, the Rac/RhoA system, as described in neutrophils, the Par/Scribble system, as described in T cells, and the segregation of L- and U-rafts, as described in T cells and neutrophils. The interrelationships between these four systems are not well defined. See the text for details.
There are many unresolved questions regarding how segregated components are integrated temporally and spatially in a cell. The answers will require technologies that recognize, quantify, and perturb local signals, as well as methods to visualize and characterize the dynamics of events that are below the resolution of the light microscope. We must obviously learn about new signaling pathways that connect the distinct circuits involved in polarization. We must also learn how, when, and where important supramolecular complexes involved in migration are formed, and quantify data on molecular dynamics and the concentrations required to achieve polarity.
Ultimately, we must develop models to study polarity and migration in physiological conditions. It is also evident that cell-cell and cell-substrate interactions are very important in the regulation of cell polarization and movement in multicellular tissues. This adds another level of complexity, one that will need further investigation, to the signaling pathways involved. New imaging, structural, and molecular technologies will be our allies in meeting these challenges.
Acknowledgements
We thank Santos Mañes lab members for their scientific contributions, and in particular R.A. Lacalle and E. Mira for critical discussions. We also thank C. Mark for editorial assistance. This work was supported in part by the Spanish Ministry of Education and Science (SAF2005-00241), Intramural CSIC founding (20050F0212) and the European Union FP6 (INNOCHEM, LSHB-CT-2005- 518167). The Department of Immunology and Oncology was founded and is supported by the Spanish National Research Council (CSIC) and by Pfizer.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/4547
References
- 1.Kohn EC, Liotta LA. Molecular insights into cancer invasion: Strategies for prevention and intervention. Cancer Res. 1995;55:1856–1862. [PubMed] [Google Scholar]
- 2.Mañes S, Mira E, Gómez-Moutón C, Lacalle RA, Martínez AC. Cells on the move: A dialogue between polarization and motility. IUBMB Life. 2000;49:89–96. doi: 10.1080/15216540050022386. [DOI] [PubMed] [Google Scholar]
- 3.Mellado M, Rodriguez-Frade JM, Mañes S, Martínez AC. Chemokine signaling and functional responses: The role of receptor dimerization and TK pathway activation. Annu Rev Immunol. 2001;19:397–421. doi: 10.1146/annurev.immunol.19.1.397. [DOI] [PubMed] [Google Scholar]
- 4.Brooks PC, Klemke RL, Schon S, Lewis JM, Schwartz MA, Cheresh DA. Insulin-like growth factor receptor cooperates with integrin alpha v beta 5 to promote tumor cell dissemination in vivo. J Clin Invest. 1997;99:1390–1398. doi: 10.1172/JCI119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chicoine MR, Silbergeld DL. Mitogens as motogens. J Neurooncol. 1997;35:249–257. doi: 10.1023/a:1005808315821. [DOI] [PubMed] [Google Scholar]
- 6.Mañes S, Llorente M, Lacalle RA, Gómez-Moutón C, Kremer L, Mira E, Martínez AC. The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in DU-145 carcinoma cells. J Biol Chem. 1999;274:6935–6945. doi: 10.1074/jbc.274.11.6935. [DOI] [PubMed] [Google Scholar]
- 7.Mira E, Lacalle RA, Gonzalez MA, Gómez-Moutón C, Abad JL, Bernad A, Martínez AC, Mañes S. A role for chemokine receptor transactivation in growth factor signaling. EMBO Rep. 2001;2:151–156. doi: 10.1093/embo-reports/kve027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zigmond S. Chemotaxis by polymorphonuclear leukocytes. J Cell Biol. 1978;77:269–287. doi: 10.1083/jcb.77.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neer EJ. Heterotrimeric G proteins: Organizers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 10.Neptune ER, Iiri T, Bourne HR. Galphai is not required for chemotaxis mediated by Gi-coupled receptors. J Biol Chem. 1999;274:2824–2828. doi: 10.1074/jbc.274.5.2824. [DOI] [PubMed] [Google Scholar]
- 11.Wong M, Fish EN. RANTES and MIP-1alpha activate stats in T cells. J Biol Chem. 1998;273:309–314. doi: 10.1074/jbc.273.1.309. [DOI] [PubMed] [Google Scholar]
- 12.Soriano SF, Serrano A, Hernanz-Falcon P, Martin de Ana A, Monterrubio M, Martinez C, Rodriguez-Frade JM, Mellado M. Chemokines integrate JAK/STAT and G-protein pathways during chemotaxis and calcium flux responses. Eur J Immunol. 2003;33:1328–1333. doi: 10.1002/eji.200323897. [DOI] [PubMed] [Google Scholar]
- 13.Sotsios Y, Ward SG. Phosphoinositide 3-kinase: A key biochemical signal for cell migration in response to chemokines. Immunol Rev. 2000;177:217–235. doi: 10.1034/j.1600-065x.2000.17712.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE. Janus kinase 2 is involved in stromal cell-derived factor-1alpha-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood. 2001;97:3342–3348. doi: 10.1182/blood.v97.11.3342. [DOI] [PubMed] [Google Scholar]
- 15.Moriguchi M, Hissong BD, Gadina M, Yamaoka K, Tiffany HL, Murphy PM, Candotti F, O'Shea JJ. CXCL12 signaling is independent of Jak2 and Jak3. J Biol Chem. 2005;280:17408–17414. doi: 10.1074/jbc.M414219200. [DOI] [PubMed] [Google Scholar]
- 16.Pollard T, Borisy G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 17.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Ridley AJ. Rho family proteins: Coordinating cell responses. Trends Cell Biol. 2001;11:471–477. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- 19.Stradal T, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 2004;14:303–311. doi: 10.1016/j.tcb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Nobes C, Hall A. Rho, rac and cdc42 GTPases: Regulators of actin structures, cell adhesion and motility. Biochem Soc Trans. 1995;23:456–459. doi: 10.1042/bst0230456. [DOI] [PubMed] [Google Scholar]
- 21.Itoh R, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol. 2002;22:6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D. Directional sensing requires G βγ-mediated PAK1 and PIX α-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 23.Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 24.Eddy R, Pierini L, Maxfield F. Microtubule asymmetry during neutrophil polarization and migration. Mol Biol Cell. 2002;13:4470–4483. doi: 10.1091/mbc.E02-04-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campello S, Lacalle RA, Bettella M, Mañes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203:2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen W, Zicha D, Ridley A, Jones G. A role for Cdc42 in macrophage chemotaxis. J Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig R, Woodhead JL. Structure and function of myosin filaments. Curr Opin Struct Biol. 2006;16:204–212. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Vicente-Manzanares M, Sánchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 2004;4:110–122. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- 30.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 31.Wong K, Pertz O, Hahn K, Bourne H. Neutrophil polarization: Spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc Natl Acad Sci USA. 2006;103:3639–3644. doi: 10.1073/pnas.0600092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worthylake R, Lemoine S, Watson J, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–160. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leeuwen FN, Kain HE, Kammen RA, Michiels F, Kranenburg OW, Collard JG. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 35.Gardiner EM, Pestonjamasp KN, Bohl BP, Chamberlain C, Hahn KM, Bokoch GM. Spatial and temporal analysis of Rac activation during live neutrophil chemotaxis. Curr Biol. 2002;12:2029–2034. doi: 10.1016/s0960-9822(02)01334-9. [DOI] [PubMed] [Google Scholar]
- 36.Sun CX, Downey GP, Zhu F, Koh AL, Thang H, Glogauer M. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood. 2004;104:3758–3765. doi: 10.1182/blood-2004-03-0781. [DOI] [PubMed] [Google Scholar]
- 37.Pestonjamasp KN, Forster C, Sun C, Gardiner EM, Bohl B, Weiner O, Bokoch GM, Glogauer M. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814–2820. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol. 2006;174:437–445. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 40.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 41.Durand CA, Westendorf J, Tse KW, Gold MR. The Rap GTPases mediate CXCL13- and sphingosine1-phosphate-induced chemotaxis, adhesion, and Pyk2 tyrosine phosphorylation in B lymphocytes. Eur J Immunol. 2006;36:2235–2249. doi: 10.1002/eji.200535004. [DOI] [PubMed] [Google Scholar]
- 42.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–928. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 43.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J Cell Biol. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJ. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Cell Biol. 2006;26:643–653. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 46.Katagiri K, Ohnishi N, Kabashima K, Iyoda T, Takeda N, Shinkai Y, Inaba K, Kinashi T. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol. 2004;5:1045–1051. doi: 10.1038/ni1111. [DOI] [PubMed] [Google Scholar]
- 47.Del Pozo M, Kiosses W, Alderson N, Meller N, Hahn K, Schwartz M. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol. 2002;4:232–239. doi: 10.1038/ncb759. [DOI] [PubMed] [Google Scholar]
- 48.Del Pozo M, Vicente-Manzanares M, Tejedor R, Serrador J, Sánchez-Madrid F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur J Immunol. 1999;29:3609–3620. doi: 10.1002/(SICI)1521-4141(199911)29:11<3609::AID-IMMU3609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 49.Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167:111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, Zugaza JL, Fischmeister R, Lezoualch F. Crosstalk between Rap1 and Rac regulates secretion of sAPPa. Nat Cell Biol. 2003;5:633–639. doi: 10.1038/ncb1007. [DOI] [PubMed] [Google Scholar]
- 51.Chant J, Stowers L. GTPase cascades choreographing cellular behavior: Movement, morphogenesis, and more. Cell. 1995;81:1–4. doi: 10.1016/0092-8674(95)90363-1. [DOI] [PubMed] [Google Scholar]
- 52.Haugh JM, Codazzi F, Teruel M, Meyer T. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J Cell Biol. 2000;151:1269–1280. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 54.Mañes S, Gómez-Moutón C, Lacalle R, Jiménez-Baranda S, Mira E, Martínez AC. Mastering time and space: Immune cell polarization and chemotaxis. Semin Immunol. 2005;17:77–86. doi: 10.1016/j.smim.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Ridley A, Schwartz M, Burridge K, Firtel R, Ginsberg M, Borisy G, Parsons J, Horwitz A. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 56.Funamoto S, Meili R, Lee S, Parry L, Firtel R. Spatial and temporal regulation of 3-phosphoinositides by PI3-Kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 57.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 58.Lacalle RA, Gómez-Moutón C, Barber DF, Jiménez-Baranda S, Mira E, Martínez AC, Carrera AC, Mañes S. PTEN regulates motility but not directionality during leukocyte chemotaxis. J Cell Sci. 2004;117:6207–6215. doi: 10.1242/jcs.01545. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 60.Nishio M, Watanabe K, Sasaki J, Taya C, Takasuga S, Iizuka R, Balla T, Yamazaki M, Watanabe H, Itoh R, Kuroda S, Horie Y, Forster I, Mak TW, Yonekawa H, Penninger JM, Kanaho Y, Suzuki A, Sasaki T. Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nat Cell Biol. 2007;9:36–44. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- 61.Hirsch E, Katanaev V, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann M. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 62.Li Z, Jiang H, Xie W, Zhang Z, Smrcka A, Wu D. Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 63.Sasaki T, Irie-Sasaki J, Jones R, Oliveira-dos-Santos A, Stanford W, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak T, Ohashi P, Suzuki A, Penninger J. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 64.Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998;1436:127–150. doi: 10.1016/s0005-2760(98)00139-8. [DOI] [PubMed] [Google Scholar]
- 65.Nombela-Arrieta C, Lacalle RA, Montoya MC, Kunisaki Y, Megias D, Marques M, Carrera AC, Manes S, Fukui Y, Martinez AC, Stein JV. Differential requirements for DOCK2 and phosphoinositide-3-kinase gamma during T and B lymphocyte homing. Immunity. 2004;21:429–441. doi: 10.1016/j.immuni.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 66.Fox JA, Ung K, Tanlimco SG, Jirik FR. Disruption of a single Pten allele augments the chemotactic response of B lymphocytes to stromal cell-derived factor-1. J Immunol. 2002;169:49–54. doi: 10.4049/jimmunol.169.1.49. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 69.Caroni P. New EMBO members' review: Actin cytoskeleton regulation through modulation of PI(4,5)P2 rafts. EMBO J. 2001;20:4332–4336. doi: 10.1093/emboj/20.16.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ling K, Schill N, Wagoner M, Sun Y, Anderson RA. Movin' on up: The role of PtdIns(4,5)P2 in cell migration. Trends Cell Biol. 2006;16:276–284. doi: 10.1016/j.tcb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Rebecchi M, Pentyala S. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 72.Cronshaw DG, Kouroumalis A, Parry R, Webb A, Brown Z, Ward SG. Evidence that phospholipase-C-dependent, calcium-independent mechanisms are required for directional migration of T-lymphocytes in response to the CCR4 ligands CCL17 and CCL22. J Leukoc Biol. 2006;79:1369–1380. doi: 10.1189/jlb.0106035. [DOI] [PubMed] [Google Scholar]
- 73.Mouneimne G, DesMarais V, Sidani M, Scemes E, Wang W, Song X, Eddy R, Condeelis J. Spatial and temporal control of cofilin activity is required for directional sensing during chemotaxis. Curr Biol. 2006;16:2193–2205. doi: 10.1016/j.cub.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 74.Mouneimne G, Soon L, DesMarais V, Sidani M, Song X, Yip S, Ghosh M, Eddy R, Backer J, Condeelis J. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J Cell Biol. 2004;166:697–708. doi: 10.1083/jcb.200405156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fievet B, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, Arpin M. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol. 2004;164:653. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yonemura S, Matsui T, Tsukita S, Tsukita S. Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: An essential role for polyphosphoinositides in vivo. J Cell Sci. 2002;115:2569–2580. doi: 10.1242/jcs.115.12.2569. [DOI] [PubMed] [Google Scholar]
- 77.Ivetic A, Ridley A. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004;112:165–176. doi: 10.1111/j.1365-2567.2004.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faure S, Salazar-Fontana L, Semichon M, Tybulewicz V, Bismuth G, Trautmann A, Germain R, Delon J. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol. 2004;5:272–279. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Harada T, Juang YT, Kyttaris VC, Wang Y, Zidanic M, Tung K, Tsokos GC. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J Immunol. 2007;178:1938–1947. doi: 10.4049/jimmunol.178.3.1938. [DOI] [PubMed] [Google Scholar]
- 80.Rameh L, Tolias K, Duckworth B, Cantley L. A new pathway for synthesis of phosphatidylinositol-4,5-bis-phosphate. Nature. 1997;390:192–196. doi: 10.1038/36621. [DOI] [PubMed] [Google Scholar]
- 81.Ishihara H, Shibasaki Y, Kizuki N, Wada T, Yazaki Y, Asano T, Oka Y. Type I phosphatidylinositol-4-phosphate 5-kinases: Cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. J Biol Chem. 1998;273:8741–8748. doi: 10.1074/jbc.273.15.8741. [DOI] [PubMed] [Google Scholar]
- 82.Kisseleva M, Feng Y, Ward M, Song C, Anderson RA, Longmore GD. The LIM protein Ajuba regulates phosphatidylinositol 4,5-bisphosphate levels in migrating cells through an interaction with and activation of PIPKI alpha. Mol Cell Biol. 2005;25:3956–3966. doi: 10.1128/MCB.25.10.3956-3966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pratt SJ, Epple H, Ward M, Feng Y, Braga VM, Longmore GD. The LIM protein Ajuba influences p130Cas localization and Rac1 activity during cell migration. J Cell Biol. 2005;168:813–824. doi: 10.1083/jcb.200406083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Margolis B, Borg JP. Apicobasal polarity complexes. J Cell Sci. 2005;118:5157–5159. doi: 10.1242/jcs.02597. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki A, Ohno S. The PAR-aPKC system: Lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 86.Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, Pasam A, Iazzolino R, Dow LE, Waterhouse NJ, Murphy A, Ellis S, Smyth MJ, Kershaw MH, Darcy PK, Humbert PO, Russell SM. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 87.Bilder D. Epithelial polarity and proliferation control: Links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 88.Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lecine P, Bellaiche Y, Dupont JL, Premont RT, Sempere C, Strub JM, VanDorsselaer A, Vitale N, Borg JP. Mammalian Scribble forms a tight complex with the βPIX exchange factor. Curr Biol. 2004;14:987–995. doi: 10.1016/j.cub.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 89.Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- 90.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 91.Johansson A, Driessens M, Aspenstrom PT. The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases Cdc42 and Rac1. J Cell Sci. 2000;113:3267–3275. doi: 10.1242/jcs.113.18.3267. [DOI] [PubMed] [Google Scholar]
- 92.Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7:262–269. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- 93.Zhang H, Macara IG. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol. 2006;8:227–237. doi: 10.1038/ncb1368. [DOI] [PubMed] [Google Scholar]
- 94.Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol. 2005;170:895–901. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 96.Ledesma MD, Brugger B, Bunning C, Wieland FT, Dotti CG. Maturation of the axonal plasma membrane requires upregulation of sphingomyelin synthesis and formation of protein-lipid complexes. EMBO J. 1999;18:1761–1771. doi: 10.1093/emboj/18.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- 98.Mañes S, Lacalle RA, Gómez-Moutón C, Martínez AC. From rafts to crafts: Membrane asymmetry in moving cells. Trends Immunol. 2003;24:320–326. doi: 10.1016/s1471-4906(03)00137-6. [DOI] [PubMed] [Google Scholar]
- 99.Mañes S, Martínez AC. Cholesterol domains regulate the actin cytoskeleton at the leading edge of moving cells. Trends Cell Biol. 2004;14:275–358. doi: 10.1016/j.tcb.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 100.Mañes S, Mira E, Gómez-Moutón C, Lacalle RA, Keller P, Labrador JP, Martínez AC. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 1999;18:6211–6220. doi: 10.1093/emboj/18.22.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mañes S, Viola A. Lipid rafts in lymphocyte activation and migration. Mol Membr Biol. 2006;23:59–69. doi: 10.1080/09687860500430069. [DOI] [PubMed] [Google Scholar]
- 102.Jiao X, Zhang N, Xu X, Oppenheim J, Jin T. Ligand-induced partitioning of human CXCR1 chemokine receptors with lipid raft microenvironments facilitates G-protein-dependent signaling. Mol Cell Biol. 2005;25:5752–5762. doi: 10.1128/MCB.25.13.5752-5762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen D, Taub D. CXCR4 function requires membrane cholesterol: Implications for HIV infection. J Immunol. 2002;168:4121–4126. doi: 10.4049/jimmunol.168.8.4121. [DOI] [PubMed] [Google Scholar]
- 104.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, Mills M, Wanzeck J, Janowska-Wieczorek A, Ratajczak M. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 105.Xue M, Vines C, Buranda T, Cimino D, Bennett T, Prossnitz E. N-formyl peptide receptors cluster in an active raftassociated state prior to phosphorylation. J Biol Chem. 2004;279:45175–45184. doi: 10.1074/jbc.M407053200. [DOI] [PubMed] [Google Scholar]
- 106.Gómez-Moutón C, Abad JL, Mira E, Lacalle RA, Gallardo E, Jimenez-Baranda S, Illa I, Bernad A, Mañes S, Martínez AC. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci USA. 2001;98:9642–9647. doi: 10.1073/pnas.171160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parat M, Anand-Apte B, Fox P. Differential caveolin-1 polarization in endothelial cells during migration in two and three dimensions. Mol Biol Cell. 2003;14:3156–3168. doi: 10.1091/mbc.E02-11-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gómez-Moutón C, Lacalle RA, Mira E, Jiménez-Baranda S, Barber DF, Carrera AC, Martinez AC, Mañes S. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol. 2004;164:759–768. doi: 10.1083/jcb.200309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gri G, Molon B, Mañes S, Pozzan T, Viola A. The inner side of T cell lipid rafts. Immunol Lett. 2004;94:247–252. doi: 10.1016/j.imlet.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 110.Moffett S, Brown D, Linder M. Lipid-dependent targeting of G proteins into rafts. J Biol Chem. 2000;275:2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- 111.Oh P, Schnitzer J. Segregation of heterotrimeric G proteins in cell surface microdomains: G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell. 2001;12:685–698. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Golub T, Caroni P. PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J Cell Biol. 2005;169:151–165. doi: 10.1083/jcb.200407058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.delPozo M, Alderson N, Kiosses W, Chiang H, Anderson RA, Schwartz M. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 114.Lacalle R, Mira E, Gómez-Moutón C, Jiménez-Baranda S, Martínez AC, Mañes S. Specific SHP-2 partitioning in raft domains triggers integrin-mediated signaling via Rho activation. J Cell Biol. 2002;157:277–289. doi: 10.1083/jcb.200109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nguyen D, Giri B, Collins G, Taub D. Dynamic reorganization of chemokine receptors, cholesterol, lipid rafts, and adhesion molecules to sites of CD4 engagement. Exp Cell Res. 2005;304:559–569. doi: 10.1016/j.yexcr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 116.Vasanji A, Ghosh P, Graham L, Eppell S, Fox P. Polarization of plasma membrane microviscosity during endothelial cell migration. Dev Cell. 2004;6:29–41. doi: 10.1016/s1534-5807(03)00397-6. [DOI] [PubMed] [Google Scholar]
- 117.Byfield F, Aranda-Espinoza H, Romanenko V, Rothblat G, Levitan I. Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophys J. 2004;87:3336–3343. doi: 10.1529/biophysj.104.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brown M, Thurmond R, Dodd S, Otten D, Beyer K. Elastic deformation of membrane bilayers probed by deuterium NMR relaxation. J Am Chem Soc. 2002;124:8471–8484. doi: 10.1021/ja012660p. [DOI] [PubMed] [Google Scholar]
- 119.Liu AP, Fletcher DA. Actin polymerization serves as a membrane domain switch in model lipid bilayers. Biophys J. 2006;91:4064–4070. doi: 10.1529/biophysj.106.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]


