Abstract
Angiogenesis, or the formation of new blood vessels from preexisting ones, is essential to establish the vascular circuit during embryonic development. During angiogenic sprouting, endothelial cells exhibit a diverse array of cellular behaviors. Endothelial tip cells must migrate extensively and proliferate in response to proangiogenic cues while trailing cells need to maintain their position and connection to the patent vasculature, despite exposure to the same proangiogenic molecules. Several new studies have now shed light on the underlying mechanisms that are responsible for coordinating this process. In particular, this work has identified a conserved role for the Notch signalling pathway in limiting the cellular angiogenic response, in part by reducing the level of vascular endothelial growth factor receptors in endothelial cells. In this overview, we discuss the emerging concepts elucidated by these studies and propose a model in which Notch acts reiteratively throughout the angiogenic process, likely by acting as a switch to determine a cell's response to Vegf.
Key words: blood vessel, angiogenesis, Notch, VEGF, zebrafish
In the developing embryo, angiogenesis plays an important role for the establishment of a functional circulatory system.1 Typically, this process entails the sprouting of endothelial cells from preexisting blood vessels. During angiogenesis, endothelial cells within a growing blood vessel sprout display distinct behaviors: cells that initially respond to pro-angiogenic signals exhibit extensive migration and proliferation as they sprout from a patent blood vessel (Fig. 1A). Subsequently, trailing cells enter the growing blood vessel sprout, yet must maintain their connection to the patent vascular network and therefore reduce their migratory response to the same pro-angiogenic cues. Similarly, most cells remain in the patent vessel and do not display any angiogenic behavior (Fig. 1B). Recent evidence from both zebrafish and mouse indicates that the Vascular Endothelial Growth Factor (Vegf)2 and Notch3 signalling pathways play critical roles to specify these distinct cell behaviors during this process.
Figure 1.
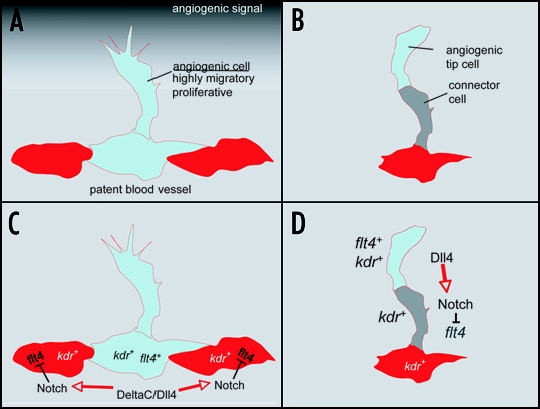
Influence of Notch signaling on angiogenesis and VEGF signaling. (A) Patent blood vessels are exposed to proangiogenic stimuli that initiate the formation of new blood vessels through angiogenesis. However, only a limited number of cells respond to these proangiogenic cues, suggesting the existence of mechanisms by which these behaviours can be reduced in neighboring cells. (B) Similar to the initiation of angiogenesis, throughout sprout development the tip cell continues to migrate while the following connector cell must remain attached to the patent vessel. These distinct behaviours are apparent despite the fact that both cells are subjected to similar proangiogenic signals from the surrounding environment (C and D). Model of tip cell specification and angiogenic sprouting in the zebrafish. (C) Notch ligands DeltaC and Dll4 in the patent blood vessel activate Notch receptors, leading to the downregulation of vegf receptor-3/flt4 after the initiation of sprouting. These endothelial cells display reduced angiogenic behavior and remain in the patent blood vessel. Those cells with less Notch activation retain flt4 expression and migrate into the developing sprout. (D) In the developing blood vessel sprout, Notch signaling is activated in the connector cell, leading to the downregulation of flt4. The migratory tip cell continues to express flt4 and kdr.
A common theme from these studies is that endothelial cells within a developing blood vessel sprout utilize the Notch signaling pathway to coordinate cellular behaviors during angiogenesis.4–8 In particular, activation of Notch receptors appears to occur primarily in “connector” cells and cells that line patent stable blood vessels, such as the dorsal aorta. Furthermore, Notch activation in these cells likely occurs through direct interaction with the Notch ligand, Dll4, which is expressed in the endothelial tip cells. Accordingly, global disruption of Notch signalling or loss of dll4 leads to an increase in endothelial cells displaying tip-cell characteristics in both mouse4,8 and zebrafish embryos.5,7 Conversely, mosaic analysis in zebrafish indicates that cells in which Notch has been activated remain in patent vessels and fail to contribute to vessels undergoing angiogenesis.7 Similarly, global activation of Notch completely blocks sprouting.5,7 These results suggest that endothelial cells with activated Notch signalling remain within the patent blood vessel, while those endothelial cells in which Notch signalling does not take place, or is reduced, are destined to leave the blood vessel.
How might Notch activation lead to decreased endothelial tip cell behavior? Previous studies have shown that migration and proliferation of endothelial cells critically depend on Vascular endothelial growth factor (Vegf).9 Interestingly, Vegf receptors in both mouse and zebrafish have been detected at much higher levels in endothelial tip cells.7,10 Furthermore, Notch can regulate the levels of Vegf receptor transcript. In endothelial cell lines Notch activation down-regulates the expression of Vegf-receptor 211 (Vegfr-2, also known as Kdr)while dll4 haploinsufficiency leads to upregulation of Vegfr-2 in the retinal vasculature of neonatal mice.8 In zebrafish, the vegf receptor-3 ortholog, flt4, is restricted to segmental artery tip cells7 and its expression in endothelial cells is controlled by Notch: zebrafish embryos lacking Notch signalling exhibit ectopic and persistent flt4 expression within all endothelial cells7,12 while Notch activation completely represses its expression.12 Taken together, these findings indicate that the Notch signalling pathway limits angiogenic behavior of endothelial cells within developing blood vessel sprouts, in part through the modulation of the Vegf signalling pathway. Interestingly, Notch similarly modulates receptor tyrosine kinase signalling pathways in other developmental processes, most notably in the Drosophila tracheal system. In this case, Notch negatively regulates expression of the fibroblast growth factor receptor to limit the contribution of cells to new tracheal branches.13 Thus, the negative regulation of receptor tyrosine kinase signalling appears to be a general conserved role for the Notch pathway during tissue morphogenesis.
Given the need to regulate angiogenic sprouting at several different steps, it is likely that Notch signaling is used reiteratively throughout this process (Fig. 1C and D). First, when cells within a patent vessel are exposed to a proangiogenic signal (e.g., Vegf), only a restricted number of cells need to initiate an angiogenic program (Fig. 1C). This makes sense physiologically as the emergence of too many cells from the patent vessel would likely compromise the integrity of the vascular system. The extent of the angiogenic response would be limited by the induction of Dll4 that in turn activates Notch in neighboring cells. Accordingly, Vegf is able to induce dll4 expression in an experimental setting.6,11 In turn, Dll4 positive cells would down-regulate Vegf receptors in neighboring cells through activation of Notch thereby preventing their migration into the developing sprout (Fig 1C). Similarly, during the sprouting process itself, the migratory behavior of connector cells must be limited to retain a patent connection to the original blood vessel (Fig. 1D). In this case, the Dll4-positive tip cell would activate Notch in the adjacent trailing cell, thereby reducing its response to Vegf and again preventing excessive migration and proliferation.
Despite these recent insights, many open questions remain. Notably, what are the molecular effects of Notch activation on the Vegf signalling pathway? Is there a complete inhibition of Vegf signalling in these cells, or does Notch act as a switch to determine the proper cellular output? Given the role of Notch as a directtranscriptional activator, the identification of direct target genes that influence Vegf signalling will be of great interest. An additional question of interest is how cell-selective activation of Notch occurs during the sprouting process, especially given the widespread expression of Notch receptor and ligand transcripts in nearly all endothelial cells. Are there post-transcriptional mechanisms that allow for specific expression of receptor and ligand protein? Are proteins known to play a role in asymmetric Notch activation, such as Numb, playing a role as well? Finally, the studies reviewed here focus mainly on the development of arteries. Indeed, the expression of both Notch receptors and ligands is restricted to arterial endothelial cells.12,14,15 This raises the question of what signals coordinate sprouting of venous endothelial cell, as well as lymphatic cells. Will there be different pathways to control this process in veins? Future studies that take advantage of both the mouse and zebrafish models will undoubtedly provide answers to these and other questions surrounding the dynamic relationship between Notch and Vegf during blood vessel development.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/4488
References
- 1.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 4.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 5.Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- 6.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 8.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 10.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 13.Ghabrial AS, Krasnow MA. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441:746–749. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- 14.Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, Kintner CR, Stark KL. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- 15.Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001;108:161–164. doi: 10.1016/s0925-4773(01)00469-5. [DOI] [PubMed] [Google Scholar]


