Abstract
Transdifferentiation is defined as the conversion of one cell type to another. It belongs to a wider class of cell type transformations called metaplasias which also includes cases in which stem cells of one tissue type switch to a completely different stem cell. Numerous examples of transdifferentiation exist within the literature. For example, isolated striated muscle of the invertebrate jellyfish (Anthomedusae) has enormous transdifferentiation potential and even functional organs (e.g., tentacles and the feeding organ (manubrium)) can be generated in vitro. In contrast, the potential for transdifferentiation in vertebrates is much reduced, at least under normal (nonpathological) conditions. But despite these limitations, there are some well-documented cases of transdifferentiation occurring in vertebrates. For example, in the newt, the lens of the eye can be formed from the epithelial cells of the iris. Other examples of transdifferentiation include the appearance of hepatic foci in the pancreas, the development of intestinal tissue at the lower end of the oesophagus and the formation of muscle, chondrocytes and neurons from neural precursor cells. Although controversial, recent results also suggest the ability of adult stem cells from different embryological germlayers to produce differentiated cells e.g., mesodermal stem cells forming ecto- or endodermally-derived cell types. This phenomenon may constitute an example of metaplasia. The current review examines in detail some well-documented examples of transdifferentiation, speculates on the potential molecular and cellular mechanisms that underlie the switches in phenotype, together with their significance to organogenesis and regenerative medicine.
Key Words: transdifferentiation, metaplasia, tissue regeneration, stem cells, plasticity, reprogramming, regenerative medicine
Introduction
Transdifferentiation is defined as the irreversible switch of one type of differentiated cell to another.1,2 Normally dedifferentiation and cell division are essential intermediate processes in the switch in phenotype, but may not be obligatory in all cases.3 Transdifferentiation is associated with a discrete change in the programme of gene expression and there is a direct ancestor-descendant relationship between the two cell types (Fig. 1). At the molecular level, the cause of transdifferentiation is presumably a change in the expression of a master switch gene (selector or homeotic gene), whose normal function is to distinguish the two cell types in normal development (see below).
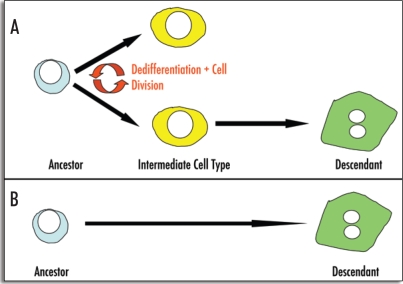
Figure 1.
Modes of transdifferentiation. Transdifferentiation can occur via different mechanisms: (A) Transdifferentiation may involve cell division and dedifferentiation prior to the adoption of a different phenotype (e.g., conversion of pigmented epithelial cells of the dorsal iris to lens fibres). The resulting intermediate cell type does not possess the phenotype of either the ancestor or the descendant cell type (B) Alternatively, transdifferentiation can occur through the direct conversion of one cell type to another without the requirement for cell division (e.g., conversion of pancreatic AR42J-B13 cells to hepatocytes).
Historically, the terms transdifferentiation and metaplasia were introduced to describe different phenomena. The term ‘transdifferentiation’ was first introduced by Selman and Kafatos to describe the transformation of the cuticle-producing cells to salt-secreting cells in the silk moth during metamorphosis from the larval to the adult moth.4 Okada and Eguchi then used the term transdifferentiation to describe the conversion of pigmented epithelial cells to lens fibres during lens regeneration in the newt. The transdifferentiation was convincingly demonstrated through utilizing an in vitro clonal cell culture system.5–7
The term ‘metaplasia’ was coined following anatomical and histological observations of the unexpected appearance of foreign tissues in ectopic sites.8 The term applies to a wider class of cell type interconversions, and is commonly used to describe a switch in cell or tissue type.9 Recently it has been used to refer to any switch of cell type regardless of pathway, which also includes interconversions between stem cells.9 Metaplasia can be found in association with tissue damage and regeneration. However, in some cases metaplasia may be due to selective outgrowth of the minor cell types originally contained in a given organ. In other cases, metaplasia may be brought about by the differentiation of stem cells, or by a switch of preexisting differentiated cells. Only the last category should be counted as true transdifferentiation, and refers to direct transformations of one differentiated cell type to another. Recently, the term plasticity has been suggested as an alternative to transdifferentiation and metaplasia, but is particularly applied to examples involving nuclear reprogramming.10
In the present review we have chosen to examine some classical and well-documented examples of metaplasia and transdifferentiation in order to illustrate the importance of the phenomenon, under normal developmental circumstances, during regeneration and in certain human pathological conditions.
Reprogramming of Adult Stem Cells for Tissue Repair and Regeneration
The classical definition of a stem cell is a type of undifferentiated cell that is both self-renewing and can generate one or more differentiated cell types given exposure to the appropriate environmental stimuli. Broadly speaking, there are two groups of stem cells, referred to as embryonic and adult (or tissue-specific) stem cells. Embryonic stem (ES) cells are normally derived from the inner cell mass of the blastocyst, and are pluripotent i.e., can give rise to many different types of differentiated cell.11 Adult or tissue specific stem cells have more limited ability to generate differentiated cells compared to embryonic stem cells. Examples of tissue-specific stem cells include haematopoietic stem cells. Haematopoietic stem cells generate all cell types of the blood and immune system. In addition to stem cells that form the blood, other organs of the mammalian body may also contain stem cells such as the skin and intestine.
Progenitor cells are the offspring of stem cells and are at an intermediate stage of differentiation. They are partly differentiated cells that divide and give rise to differentiated cells. Such cells are usually regarded as “committed” to differentiating along a particular cellular development pathway. One example of a progenitor cell is the hepatic oval cell, that resides in the cholangiocytes of the liver.12,13 When hepatocyte cell division is inhibited or impaired, the presumptive liver stem cell divides in the bile ducts and produces oval cells which are bipotential and can generate both hepatocytes and biliary epithelial cells.14
The ability of adult stems to generate cells of a particular lineage normally distinguishes adult stem cells from ES cells. There are potentially a few contradictions to this definition. We will focus on one example, that of bone marrow stem cells. Although derived from the embryonic mesoderm, the developmental potential of bone marrow stem cells may not be restricted to this germ layer and they have been shown many times to populate tissues of ectodermal and endodermal origin. Examples of such adult stem cell plasticity include bone marrow conversion to liver, kidney, lung and pancreas.15–18 These observations are contrary to the long-standing concept that tissue-specific adult stem cells are restricted to making the differentiated cell types of the tissue in which they reside. It therefore appears that bone marrow represents a possible source of pluripotent cells which might be deployed for tissue or organ repair. However, the conversion of bone marrow to other cell types is a controversial area. Some results have been hard to replicate and others have been shown to be due to cell fusion.19,20
Clinical Significance of Transdifferentiation and Metaplasia
The conversion of one cell type to another is clinically significant for two reasons. The first is because metaplasia predisposes to certain forms of neoplasia or, the development of cancer. One of the best-studied examples of this type of conversion is Barrett's metaplasia. Barrett's metaplasia (sometimes referred to as Barrett's oesophagus) is a pathological condition in which the distal region of the oesophagus undergoes a metaplastic transformation from stratified squamous to gastric or intestinal type epithelium. It is a precursor to oesophageal adenocarcinoma, a condition that has been rising rapidly in incidence in recent years.21 Estimates suggest an incidence of about 0.5–1.0% per year.22 The prognosis for oesophageal adenocarcinoma is very poor, with a median survival of less than 1 year and 5 year survival under 10%.23 So understanding the individual molecular and cellular steps leading to the appearance of ectopic intestinal tissue in the oesophagus could result either in the identification of early markers suitable for diagnostic purposes or even in the production of therapeutic strategies for curing the disease. The second reason why transdifferentiation is clinically important is related to cell-based therapies. Cellular therapies are now part of the newly emerging science of regenerative medicine. The term ‘regenerative medicine’ refers to the stimulation of regeneration of damaged or defective tissues. Regeneration of diseased or damaged organs constitutes one of the fundamental challenges to tissue engineers. The reasons for wishing to produce tissues are simple enough: due to the shortage for transplantation alternative strategies have to be found to replace diseased or damaged organs. There is currently intense interest in the field of regenerative medicine because research findings from this area may turn out to be the panacea for a spectrum of degenerative disorders including Parkinson's disease, diabetes and heart disease.
A number of possibilities exist as to the origin of the cells for replacement. Stem cells can be transplanted, which if they find the correct niche, may be able to differentiate to replace the diseased or damaged cells. An alternative possibility is to replace the missing cells or tissue by causing transdifferentiation of existing healthy cells (these could be from the patient or from a donor). The cells in theory can be generated by direct transdifferentiation in vivo or, by ex vivo approaches, using either an exogenously added factor or by gene therapy in which transdifferentiation is first induced in a culture setting and then the cells are retransplanted back into the patient.
Transdifferentiation and Organ Development
Transdifferentiation often consists of the conversion of one type of cell into another type that arose as an adjacent rudiment in the embryo.8,24 During embryogenesis, different cell types arise from a common cell sheet because different combinations of selector genes are switched on in each region in response to inductive signals. Since transdifferentiation is probably a single step change, it is logical to assume that tissues between which such changes occur are neighbors in the sense that the combination of selector genes defines the cells within them differ only in the state of one (or maybe two) genes.2,9 Where the new tissue normally consists of more than one cell type, formed from a common stem cell, the metaplastic foci usually contain all these cell types. This indicates that metaplasia represents a switch of state from one type of stem cell to another, and is distinct from the direct conversions between terminal cell types (transdifferentiation). Examples, of this type of ‘complete’ metaplasia include patches of ectopic intestinal epithelium in the stomach.25
Transdifferentiation presumably occurs because there is a change in the expression of a key transcription factor (master switch gene) that alters their state of developmental commitment. A previous theoretical work suggested that transdifferentiation might result from somatic mutation of homeotic genes normally required to distinguish tissue rudiments from one another.26 The alternative explanation is that transdifferentiation can be provoked by a change in the cellular environment and so may not involve any somatic mutation of a master switch gene. In the latter case, the master switch gene is induced by an environmental change. Assuming that stem cells in the adult have the same genotype as those in the embryo for any one cell type, then a change of state of such a gene(s) in later life would cause the stem cells to produce another cell type. Since transdifferentiation represents a switch in developmental commitment of one cell type to another, investigating the phenomenon provides a novel opportunity to explore the cellular and molecular mechanisms that may underlie specification of different organs during development. Transdifferentiation events by definition always differ from the normal sequence of development. However, understanding transdifferentiation events may also help understand normal development.
One example of cell type conversion that has been frequently cited as normally occurring is that of the change in the muscle-fibre type of the oesophagus during embryonic development.27 The oesophagus is a muscular tube that connects the pharynx to the stomach and functions as a passage to allow masticated food to enter the stomach for digestion. In the adult mouse, the main layer of muscle in the oesophagus (the muscularis externa) is composed of two layers: an inner circular layer and an outer longitudinal muscle layer. There is also an added complexity; there are different muscle fibre types in the adult oesophagus. The lower two-thirds of the mammalian oesophagus is composed almost entirely of smooth muscle, whereas the upper third is composed of skeletal muscle. The presence of the skeletal muscle is related to the fact that the first part of swallowing is under voluntary control. During early mouse development the muscle is entirely smooth muscle in character. As development proceeds, the smooth muscle in the upper third of the muscularis externa changes to skeletal type and the switch is completed by two weeks after birth. There is some debate as to whether the switch from smooth to skeletal muscle arises by transdifferentiation27,28 or by myogenesis from nonsmooth muscle cells.29
Patapoutian et al. showed that a small proportion of cells coexpressed both smooth (smooth myosin light chain) and skeletal muscle (skeletal fast myosin heavy chain) markers.27 The authors explain that the colocalization of smooth and skeletal muscle markers present from around embryonic day 16 to postnatal day 8, represented cells that possessed an intermediate phenotype and were therefore undergoing transdifferentiation. In other words, these intermediate cell types were not programmed as might be expected, had the different cell-types arisen from distinct progenitor cell populations. Rudnicki and colleagues provided support for these observations.28 Candidate factors for the skeletal muscle lineage include the family of myogenic regulatory factors (MRFs), a group of basic helix-loop helix (bHLH) transcription factors consisting of Myf5, MyoD, myogenin and MRF4.30 The four MRFs are required at different stages of muscle differentiation: MyoD and Myf5 are required for skeletal muscle determination whereas myogenin and MRF4 are required later in differentiation.31 MyoD and Myf5 knockout animals fail to undergo skeletal muscle myogenesis. Instead, the muscularis externa consist entirely of smooth muscle cells. Although this does not provide conclusive evidence for transdifferentiation, it is suggestive. Other investigations based on a Cre/loxP approach have suggested the switch in muscle type may not be an example of transdifferentiation.29 The Cre/lox technique can be applied to monitor metaplasia in vivo. The technique has been used to irreversibly label a lineage after transient activation of a tissue-specific promoter. The authors generated transgenic animals in which the smooth muscle myosin heavy chain promoter was used to drive Cre recombinase and eGFP. These mice were crossed with R26R LacZ reporter mice. In theory, any double transgenic mouse should show eGFP in smooth muscle cells and cells that were of smooth muscle origin during development will constitutively express LacZ. The results demonstrated that the skeletal muscle layer of the oesophagus does not label with LacZ. However, there are caveats to using this technique. Interpretation of the results depends heavily on the stringency or leakiness of the promoters used.
Transdifferentiation and Metaplasia During Tissue Regeneration
Metaplasia is important in the context of regeneration for two reasons. The first is because cells may arise during regeneration by metaplasia or transdifferentiation. The second is because regenerating tissues are more prone to producing cells of a different phenotype in addition to their own. Regarding the source of cells during regeneration, tissues can potentially arise by transdifferentiation or metaplasia. The source of cells for regeneration can be either undifferentiated or fully differentiated cell types. Assuming the regenerates arise from undifferentiated stem cells then this switch could be classed as a metaplasia. Alternatively, where differentiated cell types are the source of regenerates then it is possible that the source of the cells arise either from its own cell type or, from a different cell type. There are examples in the literature of regenerates arising by metaplasia and transdifferentiation some of these examples are examined below.
Urodele amphibians (e.g., the axolotl) can regenerate both limb and tail structures. A recent example of regeneration that involves cellular plasticity is tail regeneration in the axolotl.32 Tail regeneration has been reviewed in detail elsewhere.33 After tail amputation, regeneration occurs of all the structures normally found in the tail (skin, muscle, cartilage and spinal cord). Echeverri and Tanaka labelled spinal cord precursor cells by electroporating a plasmid containing GFP reporter under the control of glial acidic fibrillary protein (GFAP) promoter.32 GFAP was only expressed in the radial glial cells of the spinal cord. Using this cell lineage approach, the authors showed that during tail regeneration, neural cells (either ependymal or radial glial cells) in the spinal cord could generate muscle, chondrocytes and neurons (Fig. 2). This is a remarkable example of metaplasia since ectodermal cells are able to generate both ectodermal and mesodermal cell types, but does this occur in other vertebrates? In a recent publication, Gargioli and Slack addressed the issue of cell lineage in the regenerating Xenopus tail.34 The results showed that ‘transdifferentiation’ between differentiated cells did not occur e.g., the cells of the spinal cord and notochord regenerate from the same tissue in the stump and labeling was absent in other tissues. The question also arises as to why this type of regeneration does not occur in humans? Only by working out the differences between species that can and cannot regenerate body structures can we begin to address this problem. In the long-term, this approach could lead to the repair of severed spinal cord injury.
Figure 2.
Lineage switching of radial glial cells during axolotl tail regeneration. During tail regeneration, GFP labelled radial glial cells (green) derived from the ectoderm, switch lineage to generate muscle cells positive for myosin heavy chain (red), normally derived from the mesoderm. Reproduced with permission.22
Metaplasias generally arise in epithelial tissues when there is chronic tissue regeneration after damage caused, for example, by repeated trauma or infection.8 While some of these metaplasias (e.g., intestinal metaplasia in the stomach) will be discussed in more detail below, they do warrant a comment under the heading of regeneration. It is well known that in patients with Barrett's metaplasia, the lower end of the oesophagus becomes damaged due to reflux of the acid contents of the stomach. During regeneration of the damaged oesophageal stratified squamous epithelium from the stem cells in the oesophagus, foci of intestinal-type tissue is induced. It is likely that the persistence of metaplastic foci means that the conversion has occurred at the level of the stem cell because these cells are probably the only cells that survive in the tissue indefinitely. It is also possible that metaplastic changes may confer a selective advantage over the original cell type. For example, intestinal-type epithelium in the oesophagus may be more resistant than oesophageal epithelium to acid reflux from the stomach. During regeneration, presumably there must be a switch (e.g., induction) of a single transcription factor. In the case of intestinal metaplasia of the oesophagus, it is possible that the acid environment induces a transcription factor which reprogammes the stem cells.
Transdifferentiation and Eye Regeneration
Some amphibians demonstrate a remarkable ability to regenerate whole new structures following damage or experimental removal. In this context, one of the best characterised examples of transdifferentiation is the formation, in the adult newt, of a complete lens from the iris.35 The iris is a thin diaphragm of connective tissue and smooth muscle fibres found in front of the lens. The function of the iris is to regulate the amount of light reaching the retina. In contrast, the lens is a crystalline structure whose main function is to focus light on the retina. The developmental origin of the two eye components is quite different. The lens is formed from the epidermis, whereas the iris is formed from the optic cup, which is derived from the neuroepithelium. The process of lens regeneration from the iris pigmented epithelium is termed Wolffian regeneration, after Wolff, one of the first to investigate the phenomenon experimentally.36 Lens regeneration can also occur in the chick.37 In the newt, lens regeneration depends solely upon transdifferentiation of pigmented epithelial cells (PECs) arising from the dorsal iris. First, PECs dedifferentiate, reenter the cell cycle and proliferate. Within 10 days of lentectomy, a new lens vesicle is formed from the dedifferentiated PECs in the region of the dorsal iris. Subsequently, primary lens fibre differentiation occurs causing the lens fibres to thicken and synthesise crystallins. Gradually PEC depigmentation and proliferation declines, secondary lens fibres appear and in as little as 25–30 days following lentectomy lens regeneration is complete.38–41 Transdifferentiation of PECs to lens fibres relies in part upon the activation of thrombin, a serine protease involved in the clotting cascade.42 Thrombin is specifically activated in the dorsal iris but not in the ventral iris nor in the iris of species that cannot regenerate the lens (e.g., salamanders). Thrombin is absolutely required for PECs to reenter the cell cycle. It has been proposed that thrombin activates a yet unknown serum factor which in turn can promote dedifferentiation and proliferation of PECs.43
Lens regeneration also occurs in the frog, again through a process of transdifferentiation. Compared with the newt, there are two important differences in Xenopus lens regeneration. First, the transdifferentiating cells arise from the inner layer of the outer cornea and second, the conversion can only take place prior to metamorphosis.44 Several genes have been shown to be expressed in both the developing and regenerating lens. These genes include the fibroblast growth factor receptor 1 (fgfr1), retinoic acid receptor (rar) and the transcription factors Pax6 and Prox1.45–48 Interestingly, newt PECs and frog outer cornea are induced to transdifferentiate in vitro when cultured in the presence of FGF1, while lens regeneration in vivo is prevented by the fgfr1 specific inhibitor SU5402.49,50 Combined, these data strongly support a role for FGF1 signalling through fgfr1 as an essential mediator of the transdifferentiation events occurring in lens regeneration.
Retinal regeneration has been observed in several species and is brought about by both transdifferentiation and the activity of stem cells depending on the organism and the stage of their developing or adult life.40 As with lens regeneration, only certain urodeles possess the ability to regenerate the retina in adult life entirely through transdifferentiation.35 Retinal pigment epithelial (RPE) cells gradually become depigmented, detach from the basement membrane and adopt a transitional state where they possess both RPE and neuronal properties. The transdifferentiating rPECs are capable of self-renewal and give rise to a neuroepithelial layer of cells which differentiates to produce all retinal cell types, a process that recapitulates embryonic retinal development from the neural retina.51 The molecular mechanisms underlying the transdifferentiation of rPECs to neuroepithelial cells have not been characterised although there is some evidence to suggest that FGF2, laminin and heparin sulphate proteoglycans may be important.52–54
Transdifferentiation of Pancreas and Liver
Another example of transdifferentiation is provided by the liver and the pancreas. These two organs, each of multiple cell types, arise from neighboring regions of the endodermal epithelium and FGF signalling has been shown to direct the ventral pancreas to express genes for liver.55,56 Both conversion of pancreas to liver and the reverse, liver to pancreas transformation, have been documented. We will examine both phenomena in the current review.
Many animal models of pancreatic regeneration have been described over the years and like the liver, the pancreas has been shown to possess regenerative capacity. Bonner-Weir and colleagues, demonstrated that considerable regeneration of both endocrine and exocrine pancreatic tissue occurs in partially pancreatectomised rats only 8–10 weeks following removal of 90% of the pancreas. The response requires the recruitment of a stem cell population residing in the ductal system and proliferation of acinar cells.57,58 Another model, developed by Sarvetnick and coworkers described the autoimmune destruction of pancreatic β-cells in transgenic mice expressing interferon-γ under the control of the insulin promoter.59 The ensuing pancreatic inflammation led to β-cell loss, recapitulating the destruction of islets in type 1 diabetes. However, islets were rapidly regenerated in these mice through proliferation and differentiation of ductal cells, a process closely resembling foetal islet formation.59 Clearly, studying the mechanisms mediating regeneration in these animal models may provide insights for the development of therapies for the treatment of diabetes, but one of the most striking observations made from studies thus far is the identification of transdifferentiated hepatocytes in the regenerating pancreas.60–62
The appearance of hepatocytes in adult pancreatic tissue has been well documented. Perhaps the best characterised example is the work by Reddy and colleagues.60 The in vivo model describes extensive destruction of pancreatic acinar cells through the maintenance of adult rats on a copper-depleted diet, using the copper-chelating agent triethylenetetramine tetrahydrachloride. Rats were copper depleted for 7–9 weeks before returning them to a normal diet. During the 6–8 week recovery period more than 60% of the pancreatic volume was occupied by albumin expressing hepatocytes. Under these experimental conditions loss of acinar cells was associated with the proliferation of both ductular epithelial cells and oval cells and although the mechanism is not known, it is believed that these cells transdifferentiated to form hepatocytes. The appearance of hepatic foci in the pancreas has been reported following transplantation of a cell population enriched for pancreatic epithelial progenitors into the rat liver, in transgenic mice over expressing Keratinocyte Growth Factor in the pancreas, and as a naturally occurring phenomenon in the vervet monkey and in human pancreatic tumors.61–64
Although the in vivo models for the transdifferentiation of liver to pancreas (particularly the copper-depletion-repletion model) have been extremely valuable and demonstrate the potential for conversion of pancreas to liver, it is more difficult to determine the cellular or molecular mechanisms from these studies. One alternative approach to determine whether pancreatic hepatocytes arise directly from pancreatic cells is to establish in vitro model systems by using pancreatic cells in culture. It has been demonstrated that the synthetic glucocorticoid dexamethasone (Dex) can induce the conversion of the pancreatic tumor cell line AR42J (and a subclone AR42J-B13) to hepatocytes (Fig. 3).65 AR42J cells were originally isolated from a carcinoma of an azaserine-treated rat.66–67 Under normal circumstances AR42J cells exhibit amphicrine properties, expressing markers of both exocrine (e.g., amylase) and neuroendocrine (e.g., neurofilament) phenotypes. Expression of the pancreatic digestive enzyme amylase by AR42J cells can be enhanced by short-term (48hr) culture with dexamethasone.68 AR42J cells can also display a degree of plasticity. They can be induced to produce insulin-secreting β-cells by culture with activin and HGF or betacellulin.69,70 The addition of glucagon-like peptide-1 (GLP-1) to AR42J cells also produces insulin and glucagon expressing cells.71 The ability of the AR42J cell line to produce a variety of differentiated pancreatic cell types makes it a model for pancreatic progenitor cells and a potentially interesting system to study transdifferentiation to hepatocytes. Converting pancreatic cells to hepatocytes also offers an alternative method for producing long-term hepatocyte cultures as an in vitro model for studying liver function. The hepatocytes produced by the AR42J model exhibit many of the properties of true hepatocytes, expressing a range of markers including albumin, glucose-6-phosphatase, transferrin, transthyretin and the enzymes for Phase I and II detoxification (Fig. 4).72,73
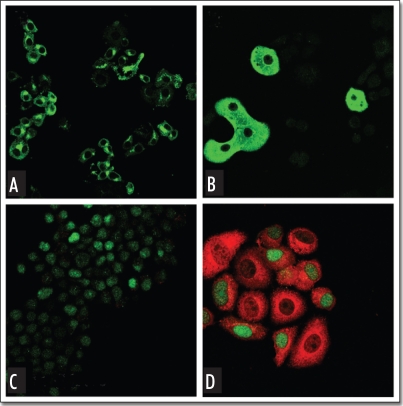
Figure 3.
(Above). Conversion of pancreatic AR42J-B13 cells to hepatocytes. Dexamethasone treatment (2 weeks) leads to the formation of flattened epithelial-like cells almost completely lacking expression of the pancreatic marker amylase (A), while the liver marker albumin (B) is induced following 3 weeks treatment with Dexamethasone. AR42J-B13 cells were transfected with nuclear GFP and stained for the liver marker glucose-6-phosphatase following 5 days in the presence of Dexamethasone. Untreated transfected B13 cells (C) do not express glucose-6-phosphatase while treated some treated cells acquire the cytoplasmic glucose-6-phosphatase staining and retain nuclear GFP (D), indicating that transdifferentiated hepatocytes arise directly from AR42J-B13 cells.55 Reproduced with permission.
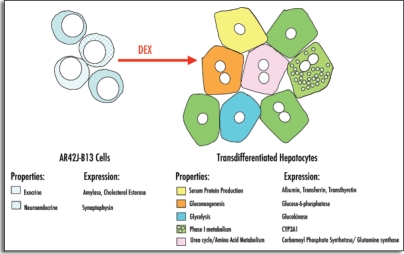
Figure 4.
(Above). Transdifferentiated hepatocytes express several markers representative of a range of hepatic functions. Pancreatic AR42J-B13 cells are amphicrine in nature expressing both exocrine and neuroendocrine properties. Following treatment with dexamethasone AR42J-B13 cells undergo a morphological change that is accompanied by induction of the expression of numerous functional hepatic markers.
The ability to induce the transdifferentiation of pancreas to liver suggests that the reverse switch should also occur readily; yet examples of this type of conversion are much more infrequent. There are some reports of the presence of pancreatic tissue in an abnormal location. Pancreatic-type tissue is found to occur in the livers of rats treated with polychlorinated biphenyls, in fish liver tumors induced by chemical carcinogens such as diethylnitrosamine, aflatoxin B1 or cyclopropenoid fatty acid, and in the liver of a human patient with hepatic cirrhosis.74–80 Intrahepatic pancreatic heterotopia has only been reported in a number of individuals, comprising less than 0.5% of all cases of heterotopic pancreas.81–83 In general, heterotopic pancreatic tissue can be composed of exocrine and/or endocrine cells. However, almost every case of pancreatic heterotopia in the liver consists entirely of exocrine cells; only one case describes the presence of endocrine cells.84 It is not known if or how there is a difference in the potential to induce exocrine and endocrine cell types in liver. In some of these examples, the hepatic exocrine tissue is most often associated with tumors or injury, such as hepatocellular carcinomas (that arise from hepatocytes), cholangiolar neoplasms (that arise from the biliary system) or adenofibrosis. Much like the human cases, these results suggest that during carcinogenesis a metaplastic event occurs that gives rise to pancreatic tissue. Indeed, pancreatic metaplasia in trout can be inhibited by the addition of the glucosinolate, indole-3-carbinol, a known anti-cancer agent.77–80
Recent evidence has shown that liver can be induced to transdifferentiate to pancreatic cells using a gene therapy approach. The gene used to convert liver to pancreas is Pancreatic and duodenal homeobox 1 (pdx1, also known as idx1, ipf1, mody4 or stf1). Pdx1 is an essential transcription factor for pancreas development. Homozygous deletion of pdx1-/- in mice results in complete loss of pancreatic tissue indicating that it is required to determine the fate of common pancreatic precursor cells and/or to regulate their propagation.85 In adult mouse pancreas, Pdx1 expression is restricted to the β-cells where it binds to and transactivates the insulin promoter.86 Specific inactivation of the pdx1 gene in β-cells results in the loss of insulin-producing cells followed by development of Type II (maturity onset) diabetes.87 Taken together, these results demonstrate the importance of Pdx1 for initial pancreas development and subsequent maintenance of the β-cell phenotype. More recently, it has been demonstrated that the liver can be induced to express a pancreatic phenotype following ectopic expression of Pdx1.88 Horb and colleagues used two models (transgenic tadpoles and human hepatoma cells) to express an activated form of Pdx1 which was able to convert liver into exocrine and endocrine pancreas.89
Recently, a study by Sumazaki and colleagues have demonstrated the conversion of the developing biliary system to pancreatic tissue in hes1 null mice.90 Although budding of the bile duct occurs from the foregut endoderm as usual, subsequent elongation of the extrahepatic and common bile ducts are abrogated. Only a truncated version of the duct is formed. These changes are associated with the appearance of exocrine and endocrine pancreatic tissue. Indeed, the full complement of endocrine cells are present in the ectopic tissue: α (glucagon), β (insulin), δ (somastostatin) and PP (pancreatic polypeptide) cells. The presence of ectopic pancreas in the developing liver may represent an example of transdetermination, or, the switch of a cell from one state of determination to another, rather than transdifferentiation, which should really only apply to fully differentiated cell types.91
Cells in both the liver and the pancreas can also, under appropriate conditions, undergo metaplasia to produce intestinal epithelium.92–95 These data support the concept that the stem cell compartments forming liver, pancreatic and intestinal epithelium are very similar, differing only in the state of one or a few selector genes.
Gastric Regenerative Epithelia and Intestinal Metaplasia
The gastric glands and crypts of Lieberkühn are the respective basic epithelium forming units of the stomach and intestine, in which reside multipotent stem cells required for the constant renewal of the gastric and intestinal epithelia. Normal gastric mucosa comprises epithelial invaginations termed pits from which tubular extensions form the glands.96 Gastric stem cells are located in the isthmus, a region near the junction between the pit and the gland. From the isthmus parietal, enteroendocrine and caveolated cells can migrate in both directions while enzyme producing zymogenic cells migrate down into the gland and mucous producing pit cells migrate towards the surface. In the intestine, stem cells located at the base of the crypts of Lieberkühn give rise to progenitors that will differentiate into secretory cells, such as enteroendocrine, goblet and Paneth cells, or cells that will become absorptive enterocytes.97 While Paneth cells differentiate at the base of the crypt where they will remain, differentiation of the other cell lineages takes place as the progenitor migrates out of the crypt toward the tip of the villi. The turnover rate of epithelial cells in both the stomach and intestine is rapid with cells being renewed every 2–7 days. Under conditions of inflammation or infection these stem cells are susceptible to genetic alteration and as a consequence metaplastic lesions may develop.
Intestinal metaplasia (or transdifferentiation), characterised by the presence of ectopic intestinal epithelia within the gastric mucosa, can be divided into two subtypes; ‘complete’ or type I intestinal metaplasia and ‘incomplete’ comprising type II and type III intestinal metaplasia.98 In ‘complete’ intestinal metaplasia ectopic tissue contains absorptive, Paneth and goblet cells, all of which are typically found in the small intestine, whereas only columnar and goblet cells are present in ‘incomplete’ intestinal metaplasia. Intestinal metaplasia is believed to predispose to gastric carcinoma, although this association is not fully accepted. Epidemiological studies have indicated that compared to individuals without, those with intestinal metaplasia have a 10-fold increased risk of developing gastric cancer with type III intestinal metaplasia carrying the greatest risk.99,100 Some studies have also shown that infection with Helicobacter pylori (H. pylori) is one of the major aetiological factors contributing to the development of intestinal metaplasia and progression through to gastric cancer.101 Persistent gastric mucosal irritation caused by H. pylori infection leads to intestinal metaplasia that is believed to arise due to the differentiation of gastric stem cells towards cells of an intestinal cell type rather than becoming cells of a gastric phenotype.
Until recently the molecular mechanism underlying this switch in fate of gastric stem cells has remained elusive. However, there are now several pieces of evidence to suggest that the caudal-related homeobox transcription factors Cdx1 and Cdx2 may play a role in the development of intestinal metaplasia. Expression of Cdx1 and Cdx2 is restricted to the epithelial layers of the small intestine and colon and in adult tissues where both are essential for the development, differentiation and maintenance of intestinal cell fate. during the later stages of mammalian development.102 Interestingly, Cdx1 and Cdx2 expression has also been localised to intestinal metaplastic tissue of human stomach indicating that ectopic expression of Cdx1/Cdx2 may contribute to the mechanism underlying intestinal metaplasia.102,103 This is supported by studies in which two independent groups generated transgenic mice mis-expressing Cdx2 in the gastric mucosa under the control of stomach specific promoters. In both cases animals were observed to develop lesions containing intestinal type tissue within the gastric mucosa.104,105 The role for Cdx2 in the maintenance of the intestinal phenotype is demonstrated in mouse studies in which there is haploinsufficiency of Cdx2.106 In addition to exhibiting axial skeletal defects due to an anterior homeotic shift, mice expressing only one allele for Cdx2 display the appearance of lesions containing forestomach epithelia (negative for Cdx2) in more posterior structures like the terminal ileum and proximal colon of the midgut. Furthermore, epimorphic regeneration of tissue types occurs between the ectopic gastric tissue and the surrounding colonic mucosa; this constitutes the first known example of this type of regeneration in mammals.
Summary and Future Perspectives
The current review has highlighted a number of well-documented examples of transdifferentiation and metaplasia. It is clear from the results of research performed both in recent years and many decades ago, that adult stem cells and differentiated cells are more versatile than was previously thought. We now know that in certain situations, genes can drive differentiated cells to a particular phenotype. For example, MyoD will convert many cell lines to muscle, and C/EBPβ will change pancreatic exocrine cells to hepatocytes.65,107 One future possibility is to identify genes that will for example, convert in a single step bone marrow to cardiomyocytes or pancreatic β-cells.
One issue related to regenerative medicine that is topical at the moment is the difference in therapeutic potential between embryonic stem cells and adult stem cells. Both have their advantages and disadvantages. For example, the differentiation of ES cells is difficult to direct in vitro. In addition, recent doubt has been cast on the claims that adult stem cells can cross germline boundaries.19,20 Irrespective of these problems, the issue is not whether either cell type can or cannot be used, but whether in the long term they can offer a cure to the patient. While the results on transdifferentiation and metaplasia from the laboratory are encouraging, it is not clear how (and when) they will translate to the bedside.
Acknowledgements
D.T. wishes to thank the Wellcome Trust, the Biotechnology and Biological Sciences Research Council and Medical Research Council for financial support.
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/abstract.php?id=1409
References
- 1.Eguchi G. Introduction: Transdifferentiation. Semin Cell Biol. 1995;6:105–108. doi: 10.1006/scel.1995.0020. [DOI] [PubMed] [Google Scholar]
- 2.Tosh D, Slack JMW. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3:187–194. doi: 10.1038/nrm761. [DOI] [PubMed] [Google Scholar]
- 3.Beresford WA. Direct transdifferentiation: Can cells change their phenotype without dividing? Cell Differ Dev. 1990;29:81–93. doi: 10.1016/0922-3371(90)90026-s. [DOI] [PubMed] [Google Scholar]
- 4.Selman K, Kafatos FC. Transdifferentiation in the labial gland of silk moths: Is DNA required for cellular metamorphosis? Cell Differ. 1974;3:81–94. doi: 10.1016/0045-6039(74)90030-x. [DOI] [PubMed] [Google Scholar]
- 5.Eguchi G, Okada TS. Differentiation of lens tissue from the progeny of chick retinal pigment cells cultured in vitro: A demonstration of a switch of cell types in clonal cell culture. Proc Natl Acad Sci USA. 1973;70:1495–1499. doi: 10.1073/pnas.70.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki M, Okada TS. Differentiation of lens and pigment cells in cultures of neural retinal cells of early chick embryos. Dev Biol. 1977;60:278–286. doi: 10.1016/0012-1606(77)90124-5. [DOI] [PubMed] [Google Scholar]
- 7.Okada TS. Transdifferentiation. Flexibility in Cell Differentiation. Oxford Clarendon Press; 1991. [Google Scholar]
- 8.Slack JMW. Epithelial metaplasia and the second anatomy. Lancet. 1986;2:268–271. doi: 10.1016/s0140-6736(86)92083-0. [DOI] [PubMed] [Google Scholar]
- 9.Slack JMW, Tosh D. Transdifferentiation and metaplasia-switching cell types. Curr Opin Genet Dev. 2001;11:581–586. doi: 10.1016/s0959-437x(00)00236-7. [DOI] [PubMed] [Google Scholar]
- 10.Pomerantz J, Blau HM. Nuclear reprogramming: A key to stem cell function in regenerative medicine. Nat Cell Biol. 2004;6:810–816. doi: 10.1038/ncb0904-810. [DOI] [PubMed] [Google Scholar]
- 11.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 12.Newsome PN, Hussain MA, Theise ND. Hepatic oval cells: Helping redefine a paradigm in stem cell biology. Curr Top Dev Biol. 2004;61:1–28. doi: 10.1016/S0070-2153(04)61001-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100:11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fausto N. Liver regeneration and repair: Hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 15.Heike T, Nakahata T. Stem cell plasticity in the hematopoietic system. Int J Hematol. 2004;79(1):7–14. doi: 10.1007/BF02983527. [DOI] [PubMed] [Google Scholar]
- 16.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 17.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Cell differentiation -Hepatocytes from nonhepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 18.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 20.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 21.Falk GW. Barrett's oesophagus. Gastroenterol. 2003;122:1569–1591. doi: 10.1053/gast.2002.33427. [DOI] [PubMed] [Google Scholar]
- 22.Jankowski JA, Provenzale D, Moayyedi P. Esophageal adenocarcinoma arising from Barrett's metaplasia has regional variations in the west. Gastroenterol. 2002;122:588–590. doi: 10.1053/gast.2002.31599. [DOI] [PubMed] [Google Scholar]
- 23.Jankowski JA, Harrison RF, Perry I, Balkwill F, Tselepis C. Barrett's metaplasia. Lancet. 2000;356:2079–2085. doi: 10.1016/S0140-6736(00)03411-5. [DOI] [PubMed] [Google Scholar]
- 24.Slack JMW. Stem cells in epithelial tissues. Science. 2000;287:1431–1433. doi: 10.1126/science.287.5457.1431. [DOI] [PubMed] [Google Scholar]
- 25.Matsukura N, Suzuki K, Kaeachi T, Aoyagi M, Sugimura T, Kitaoka H, Numajiri H, Shirota A, Habashi M, Hirota T. Distribution of maker enzymes and mucin in intestinal metaplasia in human stomach and relation of complete and incomplete metaplasia to minute gastric carcinomas. J Natl Cancer Inst. 1980;65:231–240. [PubMed] [Google Scholar]
- 26.Slack JMW. Homoeotic transformations in man: Implications for the mechanism of embryonic development and for the organization of epithelia. J Theor Biol. 1985;114:463–490. doi: 10.1016/s0022-5193(85)80179-x. [DOI] [PubMed] [Google Scholar]
- 27.Patapoutian A, Wold BJ, Wagner RA. Evidence for developmentally programmed transdifferentiation in mouse esophageal muscle. Science. 1995;270:1818–1821. doi: 10.1126/science.270.5243.1818. [DOI] [PubMed] [Google Scholar]
- 28.Kablar B, Tajbakhsh S, Rudnicki MA. Transdifferentiation of esophageal smooth to skeletal muscle is myogenic bHLH factor-dependent. Development. 2000;127:1627–1639. doi: 10.1242/dev.127.8.1627. [DOI] [PubMed] [Google Scholar]
- 29.Rishniw M, Xin H-B, Deng K-Y, Kotlikoff MI. Skeletal myogenesis in the mouse esophagus does not occur through transdifferentiation. Genesis. 2003;36:81–82. doi: 10.1002/gene.10198. [DOI] [PubMed] [Google Scholar]
- 30.Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra B, Blackwell TK, Turner D, Rupp R, Hollenberg S, Zhuang Y, Lassar A. The MyoD gene family: Nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 31.Rudnicki MA, Jaenisch R. The MyoD family of transcription factors and skeletal myogenesis. Bioessays. 1995;17:203–209. doi: 10.1002/bies.950170306. [DOI] [PubMed] [Google Scholar]
- 32.Echeverri K, Tanaka EM. Ectoderm to mesoderm lineage switching during axolotl tail regeneration. Science. 2003;298:1993–1996. doi: 10.1126/science.1077804. [DOI] [PubMed] [Google Scholar]
- 33.Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol. 2002;3:566–574. doi: 10.1038/nrm881. [DOI] [PubMed] [Google Scholar]
- 34.Gargoli C, Slack JM. Cell lineage tracing during Xenopus tail regeneration. Development. 2004;131:2669–2679. doi: 10.1242/dev.01155. [DOI] [PubMed] [Google Scholar]
- 35.Philipeaux JM. Note sur production de l'oil chez la salamandre aqauatique. Gaz Med France. 1880;51:453–457. (Fre). [Google Scholar]
- 36.Wolff G. Entwicklungsphysiologische Studien I. Die Regeneration der Urodelenlinse. German: Wilhelm Roux's Arch Entw Mechan Org. 1895;1:380–390. (Ger). [Google Scholar]
- 37.Mochii M, Mazaki Y, Mizuno N, Hayashi H, Eguchi G. Role of Mitf in differentiation and transdifferentiation of chicken pigmented epithelial cell. Dev Biol. 1998;193:47–62. doi: 10.1006/dbio.1997.8800. [DOI] [PubMed] [Google Scholar]
- 38.Eguchi G. Electron microscopic studies on lens regeneration. I. Mechanisms of depigmentation of the iris. Embryologia. 1963;8:45–62. [Google Scholar]
- 39.Eguchi G, Abe SI, Watanabe K. Differentiation of lens-like structures from newt iris epithelial cells in vitro. Natl Acad Sci USA. 1974;71:5052–5056. doi: 10.1073/pnas.71.12.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Rio-Tsonis K, Tsonis PA. Eye regeneration at a molecular age. Dev Dyn. 2003;226:211–224. doi: 10.1002/dvdy.10224. [DOI] [PubMed] [Google Scholar]
- 41.Tsonis PA, Del Rio Tsonis K. Lens and retina regeneration: Transdifferentiation, stem cells and clinical applications. Exp Eye Res. 2004;78:161–172. doi: 10.1016/j.exer.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Imokawa Y, Brockes JP. Thrombin is a critical determinant of vertebrate lens regeneration. Curr Biol. 2003;13:877–881. doi: 10.1016/s0960-9822(03)00294-x. [DOI] [PubMed] [Google Scholar]
- 43.Maden M. Regeneration: Every clot has a thrombin lining. Curr Biol. 2003;13:R517–R518. doi: 10.1016/s0960-9822(03)00444-5. [DOI] [PubMed] [Google Scholar]
- 44.Filoni S, Bernardini S, Cannata SM, D'Alessio A. Lens regeneration in larval Xenopus laevis: Experimental analysis of the regenerative capacity during development. Dev Biol. 2003;187:13–24. doi: 10.1006/dbio.1997.8598. [DOI] [PubMed] [Google Scholar]
- 45.McDevitt DS, Brahama SK, Courtois Y, Jeanny JC. Fibroblast growth factor receptors and regeneration of the eye lens. Dev Dyn. 1997;208:220–226. doi: 10.1002/(SICI)1097-0177(199702)208:2<220::AID-AJA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 46.Tsonis PA, Tsavaris M, Call MK, Chandraratna RA, Del Rio-Tsonis K. Expression and role of retinoic acid receptor alpha in lens regeneration. Dev Growth Differ. 2002;44:391–394. doi: 10.1046/j.1440-169x.2002.00652.x. [DOI] [PubMed] [Google Scholar]
- 47.Del Rio-Tsonis K, Washabaugh CH, Tsonis PA. Expression of pax-6 during urodele eye development and lens regeneration. PNAS. 1995;92:5092–5096. doi: 10.1073/pnas.92.11.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Rio-Tsonis K, Tomarev SI, Tsonis PA. Regulation of prox-1 during lens regeneration. Invest Opthalmol Vis Sci. 1999;40:2039–2045. [PubMed] [Google Scholar]
- 49.Hyuga M, Kodama R, Eguchi G. Basic fibroblast growth factor as one of the essential factors regulating lens transdifferentiation of pigmented epithelial cells. Int J Dev Biol. 1993;37:319–326. [PubMed] [Google Scholar]
- 50.Del Rio Tsonis K, Trombley MT, McMahon G, Tsonis PA. Regulation of lens regeneration by fibroblast growth factor receptor 1. Dev Dyn. 1998;213:140–146. doi: 10.1002/(SICI)1097-0177(199809)213:1<140::AID-AJA14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Morales JR, Rodrigo I, Bovolenta P. Eye development: A view from the retina pigmented epithelium. Bioessays. 2004;26(7):766–777. doi: 10.1002/bies.20064. [DOI] [PubMed] [Google Scholar]
- 52.Sakaguchi DS, Janick LM, Reh TA. Basic fibroblast growth factor (FGF2) induced transdifferentiation of retinal pigment epithelium: Generation of neurons and glia. Dev Dyn. 1997;209:387–398. doi: 10.1002/(SICI)1097-0177(199708)209:4<387::AID-AJA6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 53.Reh TA, Nagy T, Gretton H. Retinal pigmented epithelial cells induced to transdifferentiate to neurons by laminin. Nature. 1987;330:68–71. doi: 10.1038/330068a0. [DOI] [PubMed] [Google Scholar]
- 54.Nagy T, Reh TA. Inhibition of retinal regeneration in larval Rana by an antibody directed against a laminin-heparan sulfate proteoglycan. Dev Brain Res. 1994;81:131–134. doi: 10.1016/0165-3806(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 55.Wells JM, Melton DA. Vertebrate endoderm development. Ann Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- 56.Deutsch G, Jung JN, Zheng MH, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Develepment. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 57.Brockenbrough JS, Weir GC, Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes. 1988;37:232–236. doi: 10.2337/diab.37.2.232. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi K, Takahashi T, Kakita A, Yamashina S. Regional differences in the cellular proliferation activity of the regenerating rat pancreas after partial pancreatectomy. Arch Histol Cytol. 1999;62:337–346. doi: 10.1679/aohc.62.337. [DOI] [PubMed] [Google Scholar]
- 59.Sarvetnick NE, Gu D. Regeneration of pancreatic endocrine cells in interferon gamma transgenic mice. Adv Exp Med Biol. 1992;321:85–89. doi: 10.1007/978-1-4615-3448-8_10. [DOI] [PubMed] [Google Scholar]
- 60.Rao MS, Dwivedi RS, Subbarao V, Usman MI, Scarpelli DG, Nemali MR, Yeldandi A, Thangada S, Kumar S, Reddy JK. Almost total conversion of pancreas to liver in the adult rat: A reliable model to study transdifferentiation. Biochem Biophys Res Commun. 1988;156:131–136. doi: 10.1016/s0006-291x(88)80814-3. [DOI] [PubMed] [Google Scholar]
- 61.Dabeva MD, Hwang SG, Vasa SR, Hurston E, Novikoff PM, Hixson DC, Gupta S, Shafritz DA. Differentiation of pancreatic epithelial progenitor cells into hepatocytes following transplantation into rat liver. Proc Natl Acad Sci USA. 1997;94:7356–7361. doi: 10.1073/pnas.94.14.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krakowski ML, Kritzik MR, Jones EM, Krahl T, Lee J, Arnush M, Gu D, Sarvetnick N. Pancreatic expression of keratinocyte growth factor leads to differentiation of islet hepatocytes and proliferation of duct cells. Am J Pathol. 1999;154:683–689. doi: 10.1016/S0002-9440(10)65315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolfe-Coote S, Louw J, Woodroof C, Du Toit DF. The nonhuman primate endocrine pancreas: Development, regeneration potential and metaplasia. Cell Biol Int. 1996;20:95–101. doi: 10.1006/cbir.1996.0013. [DOI] [PubMed] [Google Scholar]
- 64.Paner GP, Thompson KS, Reyes CV. Hepatoid carcinoma of the pancreas. Cancer. 2000;88:1582–1589. doi: 10.1002/(sici)1097-0142(20000401)88:7<1582::aid-cncr12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 65.Shen CN, Slack JMW, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol. 2000;2:879–887. doi: 10.1038/35046522. [DOI] [PubMed] [Google Scholar]
- 66.Longnecker DS, Lilja HS, French JI, Kuhlmann E, Noll W. Transplantation of azaserine-induced carcinomas of pancreas in rats. Cancer Lett. 1979;7:197–202. doi: 10.1016/s0304-3835(79)80080-4. [DOI] [PubMed] [Google Scholar]
- 67.Christophe J. Pancreatic tumoral cell line AR42J: An amphicrine model. Am J Physiol. 1994;266:G963–G971. doi: 10.1152/ajpgi.1994.266.6.G963. [DOI] [PubMed] [Google Scholar]
- 68.Logsdon CD, Moessner J, William JA, Goldfine ID. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol. 1985;100:1200–1208. doi: 10.1083/jcb.100.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mashima H, Ohnishi H, Wakabayashi K, Mine T, Miyagawa J, Hanafusa T, Seno M, Yamada H, Kojima I. Betacellulin and Activin A corrdinately convert amylase-secreting pancreatic AR42J cells into insulin-secreting cells. J Clin Invest. 1996;97:1647–1654. doi: 10.1172/JCI118591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mashima H, Shibata H, Mine T, Kojima I. Formation of insulin-producing cells from pancreatic acinar AR42J cells by hepatocyte growth factor. Endocrinology. 1996;137:3969–3976. doi: 10.1210/endo.137.9.8756573. [DOI] [PubMed] [Google Scholar]
- 71.Zhou J, Wang X, Pineyro MA, Egan JM. Glucagon-like peptide 1 and exendin-4 convert pancreatic AR42J cells into glucagon-and insulin-producing cells. Diabetes. 1999;48:2358–2366. doi: 10.2337/diabetes.48.12.2358. [DOI] [PubMed] [Google Scholar]
- 72.Tosh D, Shen C-N, Slack JWM. Differentiated properties of hepatocytes induced from pancreatic cells. Hepatol. 2002;36:534–543. doi: 10.1053/jhep.2002.35060. [DOI] [PubMed] [Google Scholar]
- 73.Marek CJ, Cameron GA, Elrick LJ, Hawksworth GM, Wright MC. Generation of hepatocytes expressing functional cytochromes P450 from a pancreatic progenitor line in vitro. Biochem J. 2003;370:763–769. doi: 10.1042/BJ20021545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee BC, Hendricks JD, Bailey GS. Metaplastic pancreatic cells in liver tumors induced by diethylnitrosamine. Exp Mol Pathol. 1989;50:104–113. doi: 10.1016/0014-4800(89)90060-9. [DOI] [PubMed] [Google Scholar]
- 75.Rao MS, Bendayan M, Kimbrough RD, Reddy JK. Characterization of pancreatic-type tissue in the liver of rat induced by polychlorinated biphenyls. J Histochem Cytochem. 1986;34:197–201. doi: 10.1177/34.2.2418098. [DOI] [PubMed] [Google Scholar]
- 76.Wolf HK, Burchette JL, Jr, Garcia JA, Michalopoulos G. Exocrine pancreatic tissue in human liver: A metaplastic process? Amer J Surg. 1990;14:590–595. doi: 10.1097/00000478-199006000-00011. [DOI] [PubMed] [Google Scholar]
- 77.Kimbrough RD. Pancreatic-type tissue in livers of rats fed polychlorinated biphenyls. J Natl Cancer Inst. 1973;51:679–681. [PubMed] [Google Scholar]
- 78.Rao MS, Bendayan M, Kimbrough RD, Reddy JK. Characterization of pancreatic-type tissue in the liver of rat induced by polychlorinated biphenyls. J Histochem Cytochem. 1986;34:197–201. doi: 10.1177/34.2.2418098. [DOI] [PubMed] [Google Scholar]
- 79.Hendricks JD, Meyers TR, Shelton DW. Histological progression of hepatic neoplasia in rainbow trout (Salmo gairdneri) Natl Cancer Inst Monogr. 1984;65:321–336. [PubMed] [Google Scholar]
- 80.Dashwood RH, Uyetake L, Fong AT, Hendricks JD, Bailey GS. In vivo disposition of the natural anti-carcinogen indole-3-carbinol after po administration to rainbow trout. Food Chem Toxicol. 1989;27:385–392. doi: 10.1016/0278-6915(89)90144-0. [DOI] [PubMed] [Google Scholar]
- 81.Pujari BD, Deodhare SG. Intrahepatic ectopic pancreas: (Report of a case) Indian J Med Sci. 1979;33:157–159. [PubMed] [Google Scholar]
- 82.Gadrat J, Ribet A, Suduca P, Bertrand J. Pancréas aberrants intra-hépatiques. Deux cas diagnostiqués par pontion-biopsie sous contrôle laparoscopique chez deux cirrhotiques. Arch Mal Appar Dig Mal Nutr. 1965;54:1143–1148. (Fre). [PubMed] [Google Scholar]
- 83.Mobini J, Krouse TB, Cooper DR. Intrahepatic pancreatic heterotopia: Review and report of a case presenting as an abdominal mass. Am J Dig Dis. 1974;19:64–70. doi: 10.1007/BF01073355. [DOI] [PubMed] [Google Scholar]
- 84.Ballinger J. Hypoglycemia from metastasising insular carcinoma of aberrant pancreatic tissue in liver. Arch Pathol. 1941;32:277–283. [Google Scholar]
- 85.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 86.Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002;45:309–326. doi: 10.1007/s00125-001-0728-y. [DOI] [PubMed] [Google Scholar]
- 87.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes and Development. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, Benvenisti-Zarum L, Meivar-Levy I, Ferber S. Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem. 2003;278:31950–31957. doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- 89.Horb ME, Shen C-N, Tosh D, Slack JMW. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105–115. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- 90.Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- 91.Burke ZD, Shen CN, Tosh D. Bile ducts as a source of pancreatic β-cells. Bioessays. 2004;26:932–936. doi: 10.1002/bies.20090. [DOI] [PubMed] [Google Scholar]
- 92.Tatematsu M, Kaku T, Medline A, Farber E. Intestinal metaplasia as a common option of oval cells in relation to cholangiofibrosis in liver of rats exposed to 2-Acetylaminofluorene. Lab Invest. 1985;52:354–362. [PubMed] [Google Scholar]
- 93.Alison MR, Golding M, Sarraf CE, Edwards RJ, Lalani EN. Liver damage in the rat induces hepatocyte stem cells from biliary epithelial cells. Gastroenterology. 1996;110:1182–1190. doi: 10.1053/gast.1996.v110.pm8613008. [DOI] [PubMed] [Google Scholar]
- 94.Shinagawa T, Tadokoro M, Maeyama S, Maeda C, Yamaguchi S, Morohoshi T, Ishikawa E. Alpha-Fetoprotein-Producing acinar cell-carcinoma of the pancreas showing multiple lines of differentiation. Virchows Archiv-An Int J Pathol. 1995;426:419–423. doi: 10.1007/BF00191352. [DOI] [PubMed] [Google Scholar]
- 95.van Eyll JM, Pierreux CE, Lemaigre FP, Rousseau GG. Shh-dependent differentiation of intestinal tissue from embryonic pancreas by activin A. J Cell Sci. 2004;117:2077–2086. doi: 10.1242/jcs.01067. [DOI] [PubMed] [Google Scholar]
- 96.Bjerkes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Gastrointest Liver Physiol. 2002;283:G767–G777. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- 97.Stappenbeck TS, Mills JC, Gordon JI. Molecular features of adult mouse small intestinal epithelial progenitors. Proc Natl Acad Sci USA. 2003;100:1004–1009. doi: 10.1073/pnas.242735899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leung WK, Sung JJY. Review article: Intestinal metaplasia and gastric carcinogenesis. Ailment Pharmacol Ther. 2002;16:1209–1216. doi: 10.1046/j.1365-2036.2002.01300.x. [DOI] [PubMed] [Google Scholar]
- 99.Filipe MI, Munoz N, Matko I, Kato I, Pompe-Kirn V, Jutersek A, Teuchmann S, Benz M, Prijon T. Intestinal metaplasia types and the risk of gastric cancer: A cohort study of in Slovenia. Int J Cancer. 1994;57:324–329. doi: 10.1002/ijc.2910570306. [DOI] [PubMed] [Google Scholar]
- 100.You WC, Li JY, Blot WJ. Evolution of precancerous lesions in a rural chinese population at high risk of gastric cancer. Int J Cancer. 1999;83:615–619. doi: 10.1002/(sici)1097-0215(19991126)83:5<615::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 101.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Eng J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 102.Silberg DG, Furth EE, Taylor JK, Schuck T, Chiou T, Traber PG. Cdx1 protein expression in normal, metaplastic, and neoplastic human alimentary tract epithelium. Gastroenterology. 1997;113:478–486. doi: 10.1053/gast.1997.v113.pm9247467. [DOI] [PubMed] [Google Scholar]
- 103.Mizoshita T, Inada K, Tsukamoto T, Kodera Y, Yamamura Y, Hirai T, Kato T, Joh T, Itoh M, Tatematsu M. Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa-with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer. 2001;4:185–191. doi: 10.1007/pl00011741. [DOI] [PubMed] [Google Scholar]
- 104.Silberg DG, Sullivan J, Kang E, Swain GP, Moffett J, Sund NJ, Sackett SD, Kaestner KH. Cdx2 expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 105.Mutoh H, Hakamata Y, Sato K, Eda A, Yanaka I, Honda S, Osawa H, Kaneko Y, Sugano K. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Comm. 2002;294:470–479. doi: 10.1016/S0006-291X(02)00480-1. [DOI] [PubMed] [Google Scholar]
- 106.Beck F, Chawengsaksophak K, Waring P, Playford RJ, Furness JB. Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proc Natl Acad Sci USA. 1999;96:7318–7323. doi: 10.1073/pnas.96.13.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB, Miller AD. Activation of muscle specific genes in pigment, nerve, fat, liver and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci USA. 1989;86:5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]