Abstract
Many induced responses in plants are systemic. Therefore, root-induced responses may alter leaf quality for shoot herbivores. Previously, we found that root and shoot application of jasmonic acid (JA) to feral Brassica oleracea both induced glucosinolates in the leaves. However, the types of glucosinolates that increased in root- and shoot induced plants were different. Here we analyse whether primary metabolites, such as sugars and amino acids, are also differentially affected. Moreover, we test whether chemical differences in root- and shoot-induced plants differentially affect growth of the generalist Mamestra brassicae and the specialist Pieris rapae. Comprehensive analysis of glucosinolates, amino acid and sugars with principal component analysis revealed that leaf chemical profiles were affected both by JA application and by the organ that was induced. Shoot-induction increased indole glucosinolates, whereas root-induction induced aliphatic glucosinolates in the leaves. Leaves of shoot-induced plants had lower total sugar and total amino acid levels, whereas in root-induced plants only total sugar levels were significantly decreased. (Iso)leucine responded significantly different from the general trend, which allowed us to discuss the potential role of Myb transcription factors which are coordinating JA-induced glucosinolate and amino acid responses in Arabidopsis thaliana. Both M. brassicae and P. rapae grew the slowest on leaves of shoot-induced plants. M. brassicae growth and survival was also reduced on root-induced plants, whereas P. rapae growth on these plants was similar to that on controls. Specialist and generalist herbivores thus are differentially affected by the chemical changes after root and shoot-JA application.
Key words: above-below ground interactions, amino acids, Brassica oleracea, glucosinolates, induced responses, jasmonic acid, Mamestra brassicae, Pieris rapae, sugars
Introduction
Induced responses are a common phenomenon in the plant kingdom. Many plant species increase their chemical or morphological defence levels after they have been challenged by herbivores or by pathogens.1 Herbivore and pathogen attacks often do not only increase local defence levels but also induce systemic resistance in unchallenged plant parts. Similarly, systemic responses may not only occur within the shoot—thus from one leaf to another—but also from the root to the shoot and vice versa.2,3 The results of studies analyzing interactions between aboveground and belowground induced responses suggest that root-induced responses—in general—are more likely to affect shoot defence levels than the reverse.3 This implies that next to early season leaf herbivores,4 simultaneously feeding root herbivores may affect host quality for shoot herbivores as well.5
The types of—systemically—induced responses that have been observed in plants are very diverse. They range from morphological responses, e.g., increases in trichome densities, to the induction of volatiles that attract natural enemies.6,7 The specificity of the induced responses is often attributed to differences in herbivore species or feeding strategies. Pathogens, for example, elicit a different gene expression and chemical defence profile than herbivores, and aphids induce a different palette of responses than chewing caterpillars.8 It has been found that also the location of the feeding damage may affect the systemic response. In Nicotiana attenuata leaves the strength of the—systemic—protease inhibitor response depends on the ontogenetic position of the leaf that was damaged.9 As different herbivores prefer to feed on different positions in the shoots, positional effects may co-determine the nature of the defence response that is triggered.6 Such effects may also apply to root and shoot induction.
We found that application of the natural plant hormone jasmonic acid (JA)—which is generally used to elicit responses similar to those induced by chewing insect herbivores10—to the roots or to the shoots of a feral Brassica oleracea species resulted in aboveground glucosinolate induction one week after JA application.2 However, the types of glucosinolates that were induced in the leaves of root- and shoot-induced plants were completely different. Plants treated with JA to the roots mainly showed increased levels of the methionine-derived aliphatic glucosinolates in their leaves, whereas the increase in shoot-induced plants was mainly due to the tryptophan-derived indole glucosinolates.2 The biological activity of glucosinolates is closely linked to their chemical structure.11 Depending on the reaction conditions, such as pH and the presence or absence of an ethiospecifier protein, aliphatic glucosinolates give rise to (iso)thiocyanates or nitriles.12 Indole glucosinolates, on the other hand, yield unstable isothiocyanates, and their hydrolysis products are mainly nitriles, carbinols and ascorbigens.13,14 Isothiocyanates have been found to be more effective defence compounds than nitriles; Arabidopsis thaliana plants that produce mainly nitriles upon damage were found to be less resistant against the generalist Trichoplusia ni than conspecific plants producing mainly isothiocynates.15 Consequently, shoot-induced plant with increased indole glucosinolate levels in the leaves may have a different effect on herbivores than root-induced plants with increased levels of aliphatic glucosinolates.
In this study we analyse whether the differences in glucosinolate profiles between root and shoot JA-induced plants indeed differentially affect aboveground herbivores. We exposed larvae of two different lepidopteran herbivores, the generalist Mamestra brassicae and the specialist Pieris rapae, to leaves of root-and shoot induced B. oleracea plants. Based on what is known about the differences in glucosinolate profiles after root or shoot JA treatment,2 we expect that generalist herbivore performance will decrease most on leaves of root-induced plants, because of the higher levels of aliphatic glucosinolates in these plants. We also expect P. rapae to be less affected by the aliphatic glucosinolates in root-treated plants, because they have mechanisms to ‘disarm’ the plant by desulfatizing the glucosinolates or by diverting the production of isothiocyanates towards the less effective nitriles16 (but see ref. 17).
Topical application of jasmonates, however, does not exclusively induce glucosinolates but may also alter the levels of many primary compounds.18,19 First, amino acids such as tryptophan and methionine are precursors for glucosinolates. Thus increases in particular types of glucosinolates may be accompanied by changes in their amino acid precursor levels.20 Second, JA application may affect energy metabolism or photosynthetic activity and consequently the concentrations of primary metabolites in the leaves.18,19,21 Such changes may directly affect plant resistance, since primary metabolites as sugars and amino acids are known to affect herbivore growth and development.22,23 Therefore, we also analysed systemic changes in soluble amino acids and sugars in leaves of root and shoot-induced plants and correlate these to differences in herbivore performance. Moreover, by combining changes in primary and secondary chemical profiles we will obtain a more comprehensive view on the physiological mechanisms involved in root and shoot-induced responses. With this study, using quantifiable amounts of artificial induction under controlled conditions, we aim to identify factors that are of importance in more complex studies with real herbivores.
Results
Changes in chemical profiles.
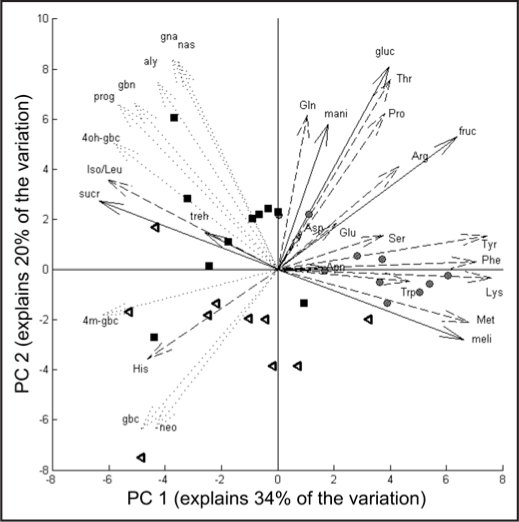
Principal component analysis (PCA) of amino acid, sugar and glucosinolates levels of the third youngest -untreated- leaf showed that the three treatment groups can be clearly separated based on their chemical profiles (Fig. 1). Control and JA-treated plants mainly separated on the first principal axis, which explained 34% of the total variation. On the one hand this separation is due to the higher levels of many sugars and amino acids in control plants (compounds with arrows pointing to the right in the figure) and on the other hand by the higher levels of glucosinolates compounds in the JA-treated plants (compounds to the left). RJA and SJA plants mainly separate on the second PC, explaining 20% of the variation. The latter separation was caused by specific differences in responses within compound classes and will be detailed below.
Figure 1.
Principal Component Analysis bi-plot of scores and loadings, based on chemical profiles of the third youngest leaf of plants at seven days after treatment with 500 µg jasmonic acid to the roots (black squares), to the shoot (open triangles) or with acidic water (control, grey circles). The length of the arrows indicates the magnitude of the loading for the variable. For amino acids (dashed lines) we used the international IUPAC three letter codes starting with a capital letter. Sugars (solid lines): gluc = glucose, fruc = fructose, mani = manitol, mel = melibiose, sucr = sucrose, treh = trehalose. For abbreviations of glucosinolates (dotted lines), see Table 1.
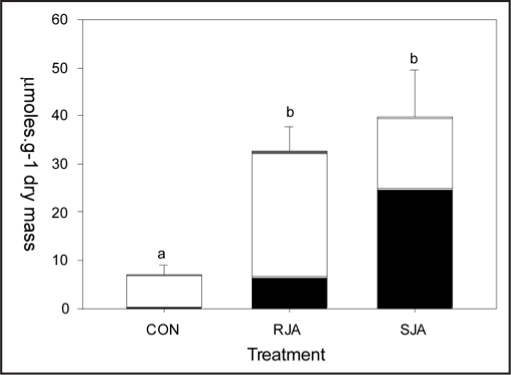
JA application increased total glucosinolate content of the third youngest—untreated—leaf, independent of whether JA was applied to the root or the shoot (Fig. 2). The profiles of the glucosinolates in these leaves, however, were significantly different between the two JA-treatments. In leaves of RJA plants, the increase in aliphatic glucosinolates was much more pronounced than in SJA plants (Fig. 2 and Table 1). As was found before in entire shoots,2 indole glucosinolates contributed significantly more to the increase in total glucosinolate levels in SJA plants than in RJA plants (Fig. 2 and Table 1). Within the group of indole glucosinolates, however, this only applied to glucobrassicin and neo-glucobrassicin and not to the compounds in the 4-hydroxy branch of the indole glucosinolate biosynthetic pathway. 4-Methoxy-glucobrassicin increased similarly in RJA and SJA plants and 4-hydroxyglucobrassicin levels were the highest in RJA plants (Table 1). This is in accordance with the position of 4-hydroxyglucobrassicin in the PCA plot (4oh-gbc), where it contributes to the separation of the RJA and SJA groups differently than the other indole glucosinolates (gbc, neo and 4m-gbc in Fig. 1).
Figure 2.
Glucosinolate levels (+SEM) in the third youngest leaf at seven days after treatment with 500 µg jasmonic acid to the roots (RJA), to the shoot (SJA) or with acidic water (CON). Black bars indicate the sum of indole glucosinolates (glucobrassicin, 4hydroxy-glucobrassicin, 4 methoxy- glucobrassicin and neoglucobrassicin), white bars are the aliphatic glucosinolates (progroitrin, glucoalyssin, gluconapin, glucobrassicanapin), and the grey bars are the aromatic glucosinolates (gluconasturtiin). Letters over the bars indicate significant differences between total glucosinolates levels between treatment groups after ANOVA (Tukey unequal N HSD, p < 0.01).
Table 1.
Glucosinolate levels (µmoles.g dry mass -1; SEM between brackets) in the third youngest leaf at seven days after treatment with 500 µg jasmonic acid to the roots (Root JA, n = 10), to the shoot (Shoot JA, n = 7) or with acidic water (Contol, n = 10)
| Biosynthetic Origin | Common Name | Code | Class | Control | Root JA | Shoot JA | ANOVA P |
| Methionine C3 | Glucoalyssin | aly | aliphatic | 1.13 (0.41)a | 4.03 (0.79)b | 1.84 (0.55)ab | 0.003 |
| Methionine C5 + AOP3 | Progoitrin | prog | aliphatic | 2.75 (0.81)a | 10.71 (1.71)b | 7.99 (2.50)ab | 0.001 |
| Methionine C4 + AOP2 | Gluconapin | gna | aliphatic | 1.50 (0.43)a | 4.95 (1.09)b | 1.83 (1.01)a | 0.004 |
| Methionine C5 + AOP2 | Glucobrassicanapin | gbn | aliphatic | 1.05 (0.33)a | 5.91 (1.07)b | 2.96 (0.92)ab | < 0.001 |
| Tryptophan | Glucobrassicin | gbc | indole | 0.25 (0.11)a | 5.83 (1.66)b | 20.16 (8.59)c | < 0.001 |
| Tryptophan | Neoglucobrassicin | neo-gbc | indole | 0.03 (0.01)a | 0.32 (0.05)a | 4.23 (1.60)b | < 0.001 |
| Tryptophan - 4OH branch | 4hydroxy-glucobrassicin | 4oh-gbc | indole | 0.08 (0.03)a | 0.29 (0.04)b | 0.19 (0.03)ab | 0.001 |
| Tryptophan - 4OH branch | 4methoxy-glucobrassicin | 4m-gbc | indole | 0.03 (0.01)a | 0.18 (0.05)b | 0.24 (0.08)b | < 0.001 |
| Phenylalanine | Gluconasturtiin | nas | aromatic | 0.17 (0.06) | 0.44 (0.08) | 0.21 (0.10) | 0.052 |
Different letters in a row indicate significant differences between treatment groups (Tukey unequal N HSD, p < 0.05 after ANOVA analysis). C3, C4, number of carbons in side chain; AOP2, AOP 3, gene involved in side chain modification, code = abbreviation in Figure 1.
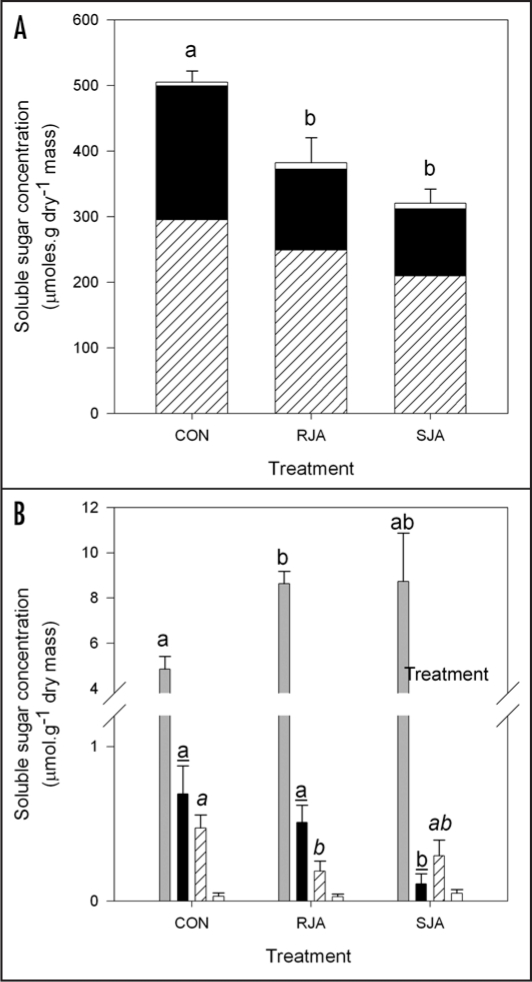
Detailed analysis of the soluble sugars in the leaves revealed that JA application systemically decreased total sugar levels independent whether JA was applied to the root or the shoot (Fig. 3A, ANOVA total sugar, treatment effect F2,26= 8.68, p = 0.001). Sucrose, however, showed a pattern exactly opposite to the general trend: its levels increased in leaves of both RJA and SJA plants (Fig. 3B, Tukey HSD CON vs. RJA, P = 0.03). Sucrose has a negative loading on the first PC, indicating that its response to JA is indeed opposite to that of the major sugars fructose and glucose, which have a positive loading (Fig. 1).
Figure 3.
Soluble sugar content (+SEM) in the third youngest leaf at seven days after treatment with 500 µg jasmonic acid to the roots (RJA), to the shoot (SJA) or with acidic water (CON). A) Major sugars. Letters over the bars indicate significant differences between total sugar levels between treatment groups after ANOVA (Tukey unequal N HSD, p < 0.03). Hatched: glucose, black fructose; white: total of minor sugars. B) Minor sugars. Letters over the bars indicate significant differences between treatment groups within sugar (Tukey unequal N HSD, p < 0.05). Grey: sucrose; black: manitol; hatched: meliobiose; white: trehalose.
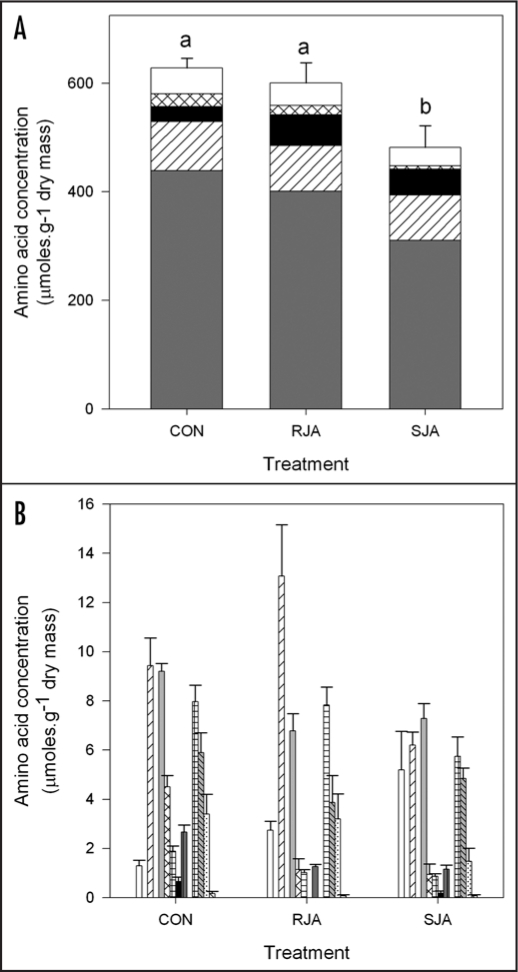
Total soluble amino acid levels in the leaves decreased significantly in SJA plants compared to RJA and control plants (Fig. 4A, ANOVA total amino acids F2,27 = 5.8, p = 0.01). Overall, individual amino acids followed this general trend with the exception of (iso)leucine and histidine (Figs. 4A and B). Compared to control plants, the levels of (iso)leucine significantly increased in RJA plants (Fig. 4A, black bars, Tukey HSD, p = 0.02), whereas histidine levels were higher in SJA plants (Fig. 4B, white bars, Tukey p = 0.003). Based on the sign of their loadings on PC 2 and the ANOVA analyses we conclude that these two amino acids are differentially affected in RJA and SJA plants (Fig. 1).
Figure 4.
Soluble amino acid content (+SEM) in the third youngest leaf at seven days after treatment with 500 µg jasmonic acid to the roots (RJA), to the shoot (SJA) or with acidic water (CON). A) Major amino acids. Letters over the bars indicate significant differences between total amino acid levels between treatment groups after ANOVA (Tukey unequal N HSD, p < 0.05). Grey: Thr; hatched: Arg; black: Iso/Leu; crossed: Pro; white: total of minor amino acids. B) Minor amino acids, separated in four groups. From left to right: Group 1 (white: His): significant increase in SJA only; Group 2 (hatched: Gln): significant decrease in SJA only; Group 3 (grey: Ser, crossed: Lys, chequered: Tyr; black: Met; dark grey: Phe): significant decrease in both JA treatments; Group 4 (chequered: Asp, grey hatched: Asn, dotted: Glu; hatched: Trp): no significant differences between treatments.
We did not find negative correlations between precursor amino acids and their respective glucosinolate biosynthetic class (methionine—aliphatic glucosinolates, tryptophan—indole glucosinolates, phenylalanine—aromatic glucosinolates). Methionine was only detected in control plants, whereas the tryptophan levels were overall too low to be detected reliably with our procedures. Phenylalanine and gluconasturtin levels were only very weakly and not significantly correlated (r = -0.17, r2 = 0.03, p = 0.39).
Effect on insect performance.
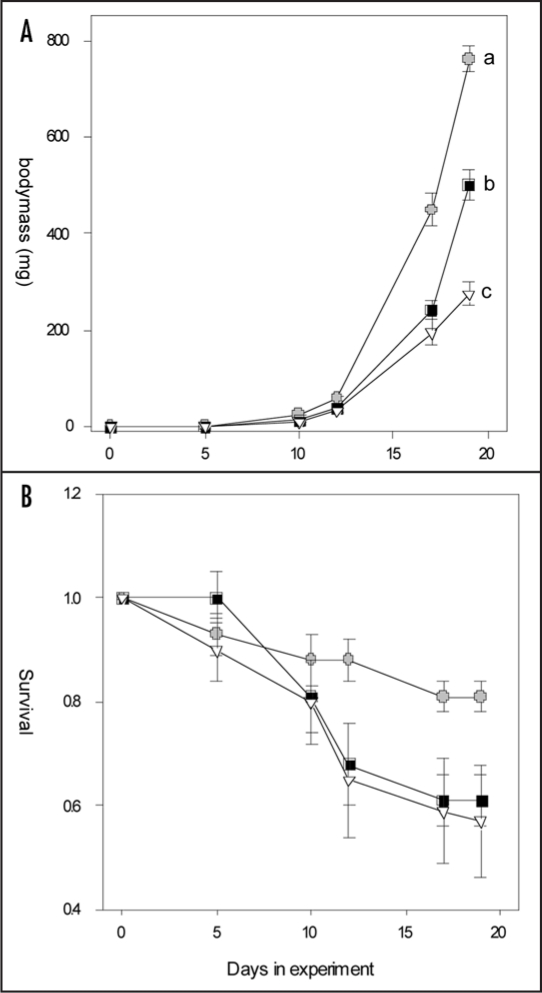
M. brassicae larvae grew significantly slower on JA-induced plants than on control plants (Fig. 5A; repeated measures ANOVA, treatment effect F2,26= 44.81, p < 0.001). Within the JA-treated plants, the larvae grew slowest on SJA plants (protected contrasts RJA vs. SJA, t = 3.79, p < 0.001). JA-induced responses reduced survival independent of where the JA was applied (Fig. 5B, Kruskall-Wallis ANOVA survival day 19, H=6.98, p = 0.03). On average 81% of the larvae survived until day 19 on control plants, whereas only 57% and 61% survived on SJA and RJA plants, respectively.
Figure 5.
Growth curves (A) and survival rate (B) of M. brassicae larvae on B. oleracea plants treated with 500 µg jasmonic acid to the shoots (open triangles), to the roots (black squares) or with acid water only (controls, grey circles). Error bars indicate SEM. Different letters indicate significant differences between growth curves after Repeated Measures ANOVA (protected contrasts, p < 0.001) or in survival rates at day 19 (Kruskall-Wallis ANOVA, p = 0.03).
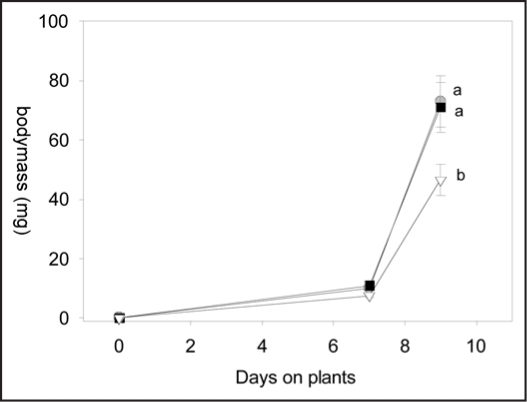
The growth of P. rapae, on the other hand, was significantly reduced on SJA plants only (Fig. 6, repeated measures ANOVA, F2,26= 5.56, p = 0.009; protected contrast RJA vs SJA, t = 2.96, p 0.006; contrast CON vs. RJA, t = 0.12, p = 0.90.). Although slightly more larvae (55%) survived in the control group than in the SJA (45%) and RJA (47.5%) groups, this difference was not statistically significant (data not shown).
Figure 6.
Growth curves of P. rapae larvae on B. oleracea plants treated with 500 µg jasmonic acid to the shoots (open triangles), to the roots (black squares) or with acid water only (controls, grey circles). Error bars indicate SEM. Different letters indicate significant differences between growth curves after Repeated Measures ANOVA (protected contrasts, p < 0.01).
Materials and Methods
Plant rearing and induction.
Seeds of feral Brassica oleracea were collected in a road side population near Heteren in 2000. A sub-set of these seeds were used to grow 10 plants in our common garden at NIOO-KNAW in Heteren for seed production in 2004. The latter seeds were used to grow plants in 1.3L pots on a peat soil-sand mixture (Potgrond 4, Lentse Potgrond B.V., Lent, NL). The pots were placed on tables in a greenhouse at 21°C (day) and 16°C (night), r.h. 60% and watered as needed. Natural daylight was supplemented with sodium lamps to maintain the minimum PAR at 225 µmol.m-2 .s-1 with a photoperiod of 16:8 (L:D).
The plants were used for experiments when they were five weeks old and had five to six true leaves. In the insect growth study (see below) the plants were replaced weekly. Therefore, every week 30 plants were grown and randomly assigned to one of the three treatment groups (n =10 per group): control (CON), root jasmonic acid application (RJA) or shoot jasmonic acid application (SJA). We used 500 µg of jasmonic acid (JA; Sigma, St Louis, MO, USA) per plant to induce our test plants to mimic induction by root and shoot herbivores. In Brassica nigra plants 500 µg JA added to the roots indeed resulted in a similar induction of glucosinolate levels after one week in the shoot as did two weeks of root fly feeding (glucosinolate levels increased 1.5 times in both experiments).2,24 The additional advantage of using JA in stead of real herbivores is that the induction treatment is qualitatively and quantitatively comparable for roots and shoots, which can not be achieved by using different species of root and shoot herbivores. Moreover, 500 µg jasmonic acid (JA) per plant, has been shown to significantly increase and differentially induce glucosinolates in B. oleracea shoots 3 to 14 days after induction;2 (Jansen JJ, van Dam NM, unpublished.).
In the RJA group, 500 µg of JA per plant was applied in 10 ml 0.1% Triton and 0.5% EtOH in water (pH = 4) by injecting the solution with a plastic syringe without needle in the soil surrounding the root-shoot interface. SJA plants were treated by gently rubbing 0.25 ml of a 2 mg/ml JA solution in 0.1% Triton and 0.5% EtOH (pH = 3.3) in water on two fully expanded leaves, usually the oldest two leaves. To control for the effects of acid application, the plant organs that were not treated with JA solution and control plants received similar amounts of 0.1% Triton and 0.5% EtOH in acidic water (pH = 3.7 with HCl) on leaves and shoots. Right after JA application, each plant received extra nutrients (50 ml Hoagland solution).
Chemical analyses.
From a separate group of 30 B. oleracea plants (CON, RJA and SJA, n = 10 each), the third youngest -untreated- leaf was sampled seven days after JA treatment and frozen at -20°C, lyophilized and stored dry and in the dark until analysis. Finely ground plant material (100.0 mg) was weighed in a 2 ml Eppendorf cup. Immediately after adding 1.0 ml 70% MeOH in water (v/v), the tube was vortexed and placed in a boiling water bath to kill remaining myrosinase activity. After 5 min. the tube was transferred to an ultrasonic bath for 15 min. and centrifuged for 10 min. at 10000 r.p.m.. The pellet was extracted again by adding 1.0 ml 70% MeOH, vortexing and 15 min. in the ultrasonic bath. Both supernatants were combined per sample in a clean and labeled 2 ml Eppendorf tube. Each tube was supplemented individually with 70% MeOH to attain the average mass (assessed with 3 tubes) of a 2 ml Eppendorf tube containing 2.0 ml 70% MeOH. This “stock” extract was stored at -20°C until further analysis. Of the ten SJA samples, three glucosinolates samples were lost due to procedural errors (n = 7 in SJA group for glucosinolate analysis).
Half (1.0 ml) of the stock extract was used for glucosinolate analysis and applied to a DEAE-Sephadex column. Further purification and glucosinolate analysis proceeded as in van Dam et al 2004.2 To calculate glucosinolate concentrations in the plant tissue, the obtained values were multiplied by 2 before dividing by dry mass.
To analyze soluble sugar content, a 10 ml aliquot of the stock extract was diluted with 990 µl MilliQ water. Soluble sugars were analyzed by injecting 5 µl of the diluted extract on Dionex HPLC system, equipped with a Carbopac PA1 column (2 x 250 mm) and a Carbopac PA1 guard column (2 x 50 mm) (Dionex Corp. Sunnyvale CA, USA). An isocratic gradient mixture of 10% 1 M NaOH and 90% MilliQ water was used to separate the sugars at a flow rate of 0.25 ml/min. Column temperature was kept at 20°C. A “10 ppm” reference solution containing 54.9 µM sorbitol and manitol, 29.21 µM trehalose, sucrose and melibiose, and 55.51 µM glucose and fructose, was diluted to obtain 7.5 ppm, 5 ppm and 2.5 ppm calibration standards to obtain a reference curve. After every 10 samples an additional standard was injected to check for deviations of retention times and the calibration curve. To calculate the original concentration in the plant material, molar sugar concentration values were multiplied by 200 before dividing them by dry mass.
For amino acid (AA) analysis 20 µl of the stock extract was diluted with 980 µl MilliQ water. AA concentration was analyzed on a Dionex HPLC system by integrated pulsed amperometric detection. A 25 µl aliquot of the diluted extract was injected and AA were separated with a ternary gradient (see DIONEX application update 152, Method 1, standard AAA gradient; condition 60/2 in) on a 2 x 250 mm AminoPac© PA10 column with a 2 x 50 mm AminoPac© PA10 Guard column (Dionex, Sunnyvale,CA, USA). Eluents, flow rates, waveform and working electrode conditions were all as specified under Method 1 in Dionex application update 152 (www1.dionex.com/en-us/webdocs/40396_AU152_V30_released°C071306.pdf) and in reference 25. The Sigma AA-S-18 amino acid standard (Sigma, St Louis, MO, USA) containing 17 AA was supplemented with aspargine, glutamine and tryptophane (2.5 µmoles/ml each) to obtain a reference sample containing the 20 most common AA. This 20 AA reference solution was diluted to obtain calibration standard ranging from 1–8 µM for each AA, except for cysteine, which had a range of 0.5–4 µM. After every 10 samples an additional standard was injected to check for deviations of retention times and the calibration curve. In our samples, isoleucine and leucine were not always sufficiently separated, so we added both values in all samples under the label iso/leu. The molar concentration of AA in the plant tissue was calculated by multiplying by 100 and dividing by dry mass.
Insect growth experiments.
We used two lepidopteran herbivores that are often reported as pest on crucifer crops in Western Europe. Mamestra brassicae (L.) (Lepidoptera: Noctuidae), or cabbage moth, is despite its common name a generalist herbivore that can severely damage cabbage and other crops.26,27 Pieris rapae (L.) (Lepidoptera: Pieridae), or the small cabbage white, is a crucifer specialist that is very common on both cultivated and wild Brassiceae.26 M. brassicae and P. rapae eggs were obtained from a culture maintained at the Laboratory of Entomology, Wageningen University, The Netherlands, on Brussels sprouts (B. oleracea var. gemmifera. cv. Cyrus). For all experiments we used neonate larvae. We performed two similar but temporally separated experiments for the two insect species.
Three days after the plants had been induced, four neonate larvae were placed on the untreated leaves of each plant (n = 40 larvae per treatment group). The larvae were allowed to move and feed freely on the shoot, but generally avoided the JA-treated leaves. To prevent the larvae from moving to another plant, the pots were placed in opaque PE sleeves that were open at both ends and fixed to the pot with a rubber band. The initial mass was assessed by weighing a representative sample of 10 additional neonate larvae on a micro-balance to the nearest µg.
In order not to disturb the larvae, the first 5 to 7 days we only assessed whether larvae were actively feeding. On day 5 (M. brassicae experiment) or day 7 (P. rapae experiment) the larvae were counted, weighed, and transferred to fresh plants that had been induced three days earlier. This was repeated at least once a week during the experiment, so that larvae always had access to sufficient leaf material of plants that were JA-induced minimally three days and maximally ten days earlier. Larvae that had died on the plants were not weighed. We only analyzed larval masses and counted survival until the first larvae had attained the (prep)pupal stage. Pupation started at day 20 for M. brassicae, and on day 11 for P. rapae.
Statistical analyses.
We analysed the relatively large multivariate chemical dataset containing both secondary and primary compound (9 glucosinolates, 6 sugars and 15 amino acids add up to 30 variables) by principal component analysis (PCA) analysis to visualize chemical profiles between treatments as well as analysis correlations between compounds. PCA analysis on all chemical data was performed using SIMCA-P (Umetrics, Umea, Sweden) software on centered and auto-scaled data. To analyse differences in individual compounds between groups in more detail, we used MANOVA protected univariate ANOVAs, i.e., ANOVAs for individual compounds were run only after MANOVA analysis showed a significant overall treatment effect for the entire compound class. The chemical data were arcsine-square root transformed before analysis. Univariate ANOVAs were followed by Tukey unequal N HSD analyses to identify significant differences between treatment groups.
Larval masses were averaged per plant and these averages were used to calculate treatment averages (n = 10 per treatment). Larval masses were log-transformed before Repeated Measures ANOVA with plant treatment as fixed effect, followed by protected contrast analyses (RJA vs. SJA, CON vs. RJA). Differences in larval survival between plant treatment groups on the last day of the experiment were analyzed with Kruskall-Wallis ANOVA. For statistical analyses other than PCA we used Statistica 7.1 (Statsoft Inc., Tusla, OK, USA) software.
Conclusions
Our analyses showed that it matters for both primary and secondary compound levels where on the plant the JA is applied. Leaves on root-induced plants showed significantly different chemical profiles than leaves on shoot-induced plants. These chemical differences elicited by root and shoot JA-application also differentially affect generalist and specialist shoot herbivores. In line with our expectations, the growth rates of the generalist M. brassicae overall were more affected by JA-induced responses than those of P. rapae. As before (ref. 2), leaves on root-induced plants had the highest levels of aliphatic glucosinolates. Unexpectedly, however, these plants were not the worst hosts for either of the herbivores tested. Both specialist as well as generalist larvae grew the slowest on shoot-induced plants, which had the highest indole glucosinolate, the lowest amino acid, and the lowest sugar levels. Possibly, the decrease in primary compounds in shoot-induced plants lowered food quality for the larvae more severely than the increase in -potentially more toxicaliphatic glucosinolates in the root-induced plants. For M. brassicae indeed a positive relation was found between larval development and nitrogen levels in the host plant.28 Similarly, it was found for P. rapae that low protein levels in its diet decreased larval mass more than increased glucosinolate levels.29 There is too little knowledge about the nutritive value of individual amino acids to draw conclusions about their specific contribution to the differences in performance of M. brassicae on root and shoot-induced plants.
Another explanation for SJA plants being of lower quality to both herbivores is that indole glucosinolates or their breakdown products have a larger detrimental effect on insect herbivores than we expected. Recently, it was found that indole glucosinolates, both in presence and absence of myrosinase, significantly reduce the growth of the generalist aphid Myzus persicae whereas the aliphatic glucosinolate sinigrin did not.30 Moreover, low concentrations of indole glucosinolates effectively induced expression of P450 detoxification enzymes in the generalist herbivore Helicoverpa zea, indicating that also these glucosinolates may act as toxins.31 It is as yet unknown how M. brassicae larvae deal with glucosinolates and other allelochemicals in their food or which detoxification enzymes they posses. P. rapae is known to possess a nitrile specifier protein (NSP) which interferes with the conversion of glucosinolates by myrosinase, resulting in the production of the less toxic nitriles in stead of isothiocyanates.15,16 NSP activity in P. rapae thus may explain why these larvae grew equally well on root-induced and control plants. However, it is unknown whether the NSP in P. rapae also plays a role in the detoxification of indole glucosinolates to the benefit of the insect.16 However, a direct detrimental effect of indole glucosinolates on P. rapae is not expected, because for this species these compounds serve as positive oviposition and feeding cues.11,32–34
Finally the reduced growth rates on shoot-induced plants may have been due to other differentially expressed defence compounds in JA-induced plants, such as protease inhibitors (PI) flavonoids, or hydroxycinnamates that are commonly induced by jasmonate application in Brassicaceae.19,21,35,36 Shoot PI levels in Nicotiana attenuata, for example, were significantly higher in shoot-induced plants that in root-induced plants.9 The performance of M. brassicae indeed may be effectively inhibited by PI.35,37 P. rapae performance or protease activity, on the other hand, were not affected by Brassica PI, which makes it unlikely that increased PI levels contributed to the reduced growth rates of this specialist caterpillar.38 Data on JA-induced changes in other defence related compounds, such as flavonoids and hydroxycinnamates, are available of shoot induced plants only.19,21 Hence we do not know whether or how the levels of these compounds may have differed differ between root and shoot JA induced plants.
For glucosinolates, we found interesting differences in JA responses both between and within main biosynthetic pathways. Root induction increased the levels of aliphatic glucosinolates more than shoot induction (Table 1). This overall increase suggest that the many genes, e.g., MAM1, CYP79F1, CYP79F2, CYP83A, AOP2 and AOP3, involved in the biosynthesis of aliphatic glucosinolates are all upregulated synchronically in leaves of root-induced plants.39,40 Recently two transcription factors that coordinate aliphatic glucosinolate biosynthetic gene activity, PMG1/Myb28 and PMG2/Myb29 have been identified by comprehensive analysis of Arabidopsis thaliana expression profiles.41 Myb28 was found to be essential for constitutive production of aliphatic glucosinolates, whereas Myb29 was responsible for MethylJA-induced increases. Most interestingly, it was found that both factors closely cluster and positively regulate genes involved in leucine biosynthesis.41 Our finding that both aliphatic glucosinolates and (iso)leucine levels increase in RJA plants (Fig. 3), corroborates the hypothesis that in RJA plants a similar Myb-factor is involved in producing the specific leaf chemical profile. Other related Myb factors as ATR1/Myb24, HIG/Myb51 and OBP2 have been identified that may be responsible for the specific induction of indole glucosinolates in SJA plants.39,42,43 Because over-expression of these Myb factors increased all indole glucosinolates in the plant, regulation by one of these Myb factors in our B. oleraceae can not explain why 4-hydroxy- and 4-methoxy-glucobrassicin are regulated differently from glucobrassicin and neoglucobrassicin.42 Interestingly it was found for most of the above Myb factors that over-expression resulted not only in an increase of the positively regulated pathway, but also in a suppression of the other glucosinolate biosynthetic pathways. This has led to the hypothesis that interactions between Myb factors and biosynthetic gene activities may maintain a ‘glucosinolate homeostasis’ which keeps the total glucosinolate level stable by alternating between pathways.44 The similar increases in total glucosinolates levels in root- and shoot-induced plants could indeed be an indicator for homeostasis between glucosinolate pathways. It should be noted, however, that in both JA treatments the levels of the less-induced glucosinolates still were significantly increased compared to controls (Table 1). More studies on the genomic level using JA-induced B. oleracea plant material are needed to determine how interactions between Myb factors and biosynthetic pathways may lead to the specific glucosinolate patterns we observed in root and shoot-induced plants of this species.
Our experiments clearly show that plants respond differently to JA induction depending on which organ is induced and that the resulting differences in chemical composition of the leaves differentially affect herbivore growth. We realize that JA application may not completely mimic responses induced by natural herbivory.45 However, our experiment indicates that root and shoot induction may be regulated in completely different ways. This calls the question how similar root- and shoot-induced signalling pathways are, how they interact and whether this interaction interferes with optimal defence induction. B. nigra infested with root flies, for example, showed increased resistance against leaf feeding herbivores, but also a lower attractiveness to natural enemies of these herbivores due to changes in volatile profiles.24,46 One of the next challenges will be to analyze how such signalling interactions occurring at the molecular level shape the chemical phenotype of plants, and how these interactions affect insect resistance as well as plant performance in natural and agricultural systems.
Acknowledgements
The authors thank Ciska Raaijmakers, Roel Wagenaar and Sylvia Lenting for their assistance with plant rearing and the chemical analyses. We thank Leo Koopman and André Gidding of Entomology, Wageningen University The Netherlands, for proving the insect eggs. Jeroen Jansen made the PCA figure. Rieta Gols, Jeff Harvey and Jeroen Jansen provided helpful comments on earlier versions of this MS. N.M. van Dam is funded by a NWO VIDI grant, no. 864-02-001, of the Netherlands Organisation for Scientific Research. Publication 4188 NIOO-KNAW Netherlands Institute of Ecology.
Abbreviations
- JA
jasmonic acid
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5220
References
- 1.Agrawal AA, Tuzun S, Bent E. Induced Plant Defenses Against Pathogens and Herbivores: Biochemistry, Ecology, and Agriculture. St. Paul, Minnesota: APS Press; 1999. [Google Scholar]
- 2.van Dam NM, Witjes L, Svatos A. Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. New Phytol. 2004;161:801–810. doi: 10.1111/j.1469-8137.2004.00984.x. [DOI] [PubMed] [Google Scholar]
- 3.Bezemer TM, van Dam NM. Above- belowground interactions via induced plant defenses. Trends Ecol Evol. 2005;20:617–624. doi: 10.1016/j.tree.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Van Zandt PA, Agrawal AA. Specificity of induced plant responses to specialist herbivores of the common milkweed Asclepias syriaca. Oikos. 2004;104:401–409. [Google Scholar]
- 5.van Dam NM, Raaijmakers CE, Van der Putten WH. Root herbivory reduces growth and survival of the shoot feeding specialist Pieris rapae on Brassica nigra. Entomol Exp Appl. 2005;115:161–170. [Google Scholar]
- 6.Traw MB, Dawson TE. Differential induction of trichomes by three herbivores of black mustard. Oecologia. 2002;131:526–532. doi: 10.1007/s00442-002-0924-6. [DOI] [PubMed] [Google Scholar]
- 7.Mattiacci L, Rudelli S, Rocca BA, Genini S, Dorn S. Systemically-induced response of cabbage plants against a specialist herbivore, Pieris brassicae. Chemoecology. 2001;11:167–173. [Google Scholar]
- 8.De Vos M, Van Oosten VR, Van Poecke RMP, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Metraux JP, Van Loon LC, Dicke M, Pieterse CMJ. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Molecular Plant-Microbe Interactions. 2005;18:923–937. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- 9.van Dam NM, Horn M, Mares M, Baldwin IT. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. Journal of Chemical Ecology. 2001;27:547–568. doi: 10.1023/a:1010341022761. [DOI] [PubMed] [Google Scholar]
- 10.Staswick PE, Lehman CC. Jasmonic acid-singaled responses in plants. In: Agrawal AA, Tuzun S, Bent E, editors. Induced Plant Defenses Against Pathagens and Herbivores. St. Paul, Minnesota: APS Press; 1999. pp. 117–136. [Google Scholar]
- 11.Chew FS. Biological effects of glucosinolates. In: Cutler HG, editor. Biologically Active Natural Products. Washington DC: ACS; 1988. pp. 155–181. [Google Scholar]
- 12.Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annual Review of Plant Biology. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- 13.Bones AM, Rossiter JT. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry. 2006;67:1053–1067. doi: 10.1016/j.phytochem.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Agerbirk N, Olsen CE, Sorensen H. Initial and final products, nitriles, and ascorbigens produced in myrosinase-catalyzed hydrolysis of indole glucosinolates. Journal of Agricultural and Food Chemistry. 1998;46:1563–1571. [Google Scholar]
- 15.Lambrix V, Reichelt M, Mitchell-Olds T, Kliebenstein DJ, Gershenzon J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell. 2001;13:2793–2807. doi: 10.1105/tpc.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wittstock U, Agerbirk N, Stauber EJ, Olsen CE, Hippler M, Mitchell-Olds T, Gershenson J, Vogel H. Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc Nat Acad Sci USA. 2004;101:4859–4864. doi: 10.1073/pnas.0308007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agrawal AA, Kurashige NS. A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J Chem Ecol. 2003;29:1403–1415. doi: 10.1023/a:1024265420375. [DOI] [PubMed] [Google Scholar]
- 18.Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annual Review of Plant Physiology and Molecular Biology. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- 19.Liang YS, Choi YH, Kim HK, Linthorst HJM, Verpoorte R. Metabolomic analysis of methyl jasmonate treated Brassica rapa leaves by 2-dimensional NMR spectroscopy. Phytochemistry. 2006;67:2503–2511. doi: 10.1016/j.phytochem.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Halkier BA, Du L. The biosynthesis of glucosinolates. Trends Plant Sci. 1997;2:425–431. doi: 10.1016/j.tplants.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Hendrawati O, Yao QQ, Kim HK, Linthorst HJM, Erkelens C, Lefeber AWM, Choi YH, Verpoorte R. Metabolic differentiation of Arabidopsis treated with methyl jasmonate using nuclear magnetic resonance spectroscopy. Plant Sci. 2006;170:1118–1124. [Google Scholar]
- 22.Soldaat LL, Vrieling K. The influence of nutritional and genetic factors on larval performance of the cinnabar moth Tyria jacobaeae. Entomol Exp Appl. 1992;62:29–36. [Google Scholar]
- 23.Bartlet E, Parsons D, Williams IH, Clark SJ. The influence of glucosinolates and sugars on feeding by the cabbage stem flea beetle, Psylloides chrysocephala. Entomol Exp Appl. 1994;73:77–83. [Google Scholar]
- 24.van Dam NM, Raaijmakers CE. Local and systemic induced responses to cabbage root fly larvae (Delia radicum) in Brassica nigra and B. oleracea. Chemoecology. 2006;16:17–24. [Google Scholar]
- 25.Hanko VP, Rohrer JS. Determination of amino acids in cell culture and fermentation broth media using anion-exchange chromatography with integrated pulsed amperometric detection. Analytical Biochemistry. 2004;324:29–38. doi: 10.1016/j.ab.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Gratwick M. Collected Edition of MAFF Leaflets. London: Chapmann and Hall; 1992. Crop Pests in the UK. [Google Scholar]
- 27.Rojas JC, Wyatt TD, Birch MC. Flight and oviposition behavior toward different host plant species by the cabbage moth, Mamestra brassicae (L.) (Lepidoptera : Noctuidae) J Insect Behav. 2000;13:247–254. [Google Scholar]
- 28.Newington JE, Setala H, Bezemer TM, Jones TH. Potential effects of earthworms on leaf-chewer performance. Functional Ecology. 2004;18:746–751. [Google Scholar]
- 29.Rotem K, Agrawal AA, Kott L. Parental effects in Pieris rapae in response to variation in food quality: Adaptive plasticity across generations? Ecol Entomol. 2003;28:211–218. [Google Scholar]
- 30.Kim JH, Jander G. Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. The Plant Journal. 2007:1008–1019. doi: 10.1111/j.1365-313X.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- 31.Li XC, Berenbaum MR, Schuler MA. Plant allelochemicals differentially regulate Helicoverpa zea cytochrome P450 genes. Insect Molecular Biology. 2002;11:343–351. doi: 10.1046/j.1365-2583.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- 32.Renwick JAA, Radke CD, Sachdev-Gupta K, Städler E. Leaf surface chemicals stimulating oviposition by Pieris rapae (Lepidoptera: Pieridae) on cabbage. Chemoecology. 1992;3:33–38. [Google Scholar]
- 33.Gouinguene SPD, Städler E. Comparison of the sensitivity of four Delia species to host and non-host plant compounds. Physiol Entomol. 2005;30:62–74. [Google Scholar]
- 34.van Loon JJA, Blaakmeer A, Griepink FC, van Beek TA, Schoonhoven LM, de Groot A. Leaf surface compound from Brassica oleracea (Cruciferae) induces oviposition by Pieris brassicae (Lepidoptera: Pieridae) Chemoecology. 1992;3:39. [Google Scholar]
- 35.De Leo F, Bonade-Bottino M, Ceci LR, Gallerani R, Jouanin L. Effects of a mustard trypsin inhibitor expressed in different plants on three lepidopteran pests. Insect Biochemistry and Molecular Biology. 2001;31:593–602. doi: 10.1016/s0965-1748(00)00164-8. [DOI] [PubMed] [Google Scholar]
- 36.De Leo F, Ceci LR, Jouanin L, Gallerani R. Analysis of mustard trypsin inhibitor-2 gene expression in response to developmental of environmental induction. Planta. 2001;212:710–717. doi: 10.1007/s004250000474. [DOI] [PubMed] [Google Scholar]
- 37.Rymerson RT, Bodnaryk RP. Gut proteinase activity in insect pests of Canola. Canadian Entomologist. 1995;127:41–48. [Google Scholar]
- 38.Broadway RM. Dietary proteinase inhibitors alter complement of midgut proteases. Archives of Insect Biochemistry and Physiology. 1996;32:39–53. [Google Scholar]
- 39.Grubb CD, Abel S. Glucosinolate metabolism and its control. Trends Plant Sci. 2006;11:89–100. doi: 10.1016/j.tplants.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Hirai MY, Klein M, Fujikawa Y, Yano M, Goodenowe DB, Yamazaki Y, Kanaya S, Nakamura Y, Kitayama M, Suzuki H, Sakurai N, Shibata D, Tokuhisa J, Reichelt M, Gershenzon J, Papenbrock J, Saito K. Elucidation of gene-to-gene and metabolite-to-gene networks in Arabidopsis by integration of metabolomics and transcriptomics. J Biol Chem. 2005;280:25590–25595. doi: 10.1074/jbc.M502332200. [DOI] [PubMed] [Google Scholar]
- 41.Hirai MY, Sugiyama K, Sawada Y, Tohge T, Obayashi T, Suzuki A, Araki R, Sakurai N, Suzuki H, Aoki K, Goda H, Nishizawa OI, Shibata D, Saito K. Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc Nat Acad Sci USA. 2007:6478–6483. doi: 10.1073/pnas.0611629104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gigolashvili T, Berger B, Mock HP, Muller C, Weisshaar B, Flugge UI. The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 2007:886–901. doi: 10.1111/j.1365-313X.2007.03099.x. [DOI] [PubMed] [Google Scholar]
- 43.Skirycz A, Reichelt M, Burow M, Birkemeyer C, Rolcik J, Kopka J, Zanor MI, Gershenzon J, Strnad M, Szopa J, Mueller-Roeber B, Witt I. DOF transcription factor AtDof1.1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant J. 2006;47:10–24. doi: 10.1111/j.1365-313X.2006.02767.x. [DOI] [PubMed] [Google Scholar]
- 44.Hemm MR, Ruegger MO, Chapple C. The Arabidopsis ref2 mutant is defective in the gene encoding CYP83A1 and shows both phenylpropanoid and glucosinolate phenotypes. Plant Cell. 2003;15:179–194. doi: 10.1105/tpc.006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cipollini DF, Sipe ML. Jasmonic acid treatment and mammalian herbivory differentially affect chemical defenses and growth of wild mustard (Brassica kaber) Chemoecology. 2001;11:137–143. [Google Scholar]
- 46.Soler R, Harvey JA, Kamp AFD, Vet LEM, Van der Putten WH, Van Dam NM, Stuefer JF, Gols R, Hordijk CA, Martijn Bezemer T. Root herbivores influence the behaviour of an aboveground parasitoid through changes in plant-volatile signals. Oikos. 2007:367–376. [Google Scholar]