Abstract
Conifers like Scots pine (Pinus sylvestris) have a complicated root system consisting of morphologically and anatomically different root types, of which the short roots have a very limited ability to elongate. Short roots have an important role in nature since they are able to establish ectomycorrhizal symbiosis, in which the growth of fungal mycelium between the epidermal cells and in the intercellular space between cortical cells leads to formation of dichotomous short roots, which may, through further splitting of the meristem, form coralloid root structures. Dichotomous short roots have been suggested to result from changes in either auxin or ethylene concentrations due to the fungal growth inside the root. NPA, the inhibitor of polar auxin transport, enhances the dichotomization of P. sylvestris short root tips similarly to the fungal growth in the root, thus confirming that auxin plays a role in short root morphogenesis. Ethylene is also known to have an important role in the regulation of root morphogenesis. In future the research dealing with the root system and ectomycorrhiza development in P. sylvestris must take into account that both auxin and ethylene are involved and that there is no contradiction in obtaining the same phenotype with both hormones. The expression analysis of PIN proteins, auxin efflux carriers, could give valuable information about the role of auxin transport in regulating the root growth and morphogenesis of coniferous root system and mycorrhiza.
Key words: auxin transport, dichotomization, ethylene, mycorrhiza, open meristem, PIN, Pinus sylvestris, short root
Introduction
Efflux-mediated auxin gradients are suggested to represent a common module that operates in the formation of all plant organs, regardless of their developmental origin and fate.1–3 Current research with Arabidopsis thaliana and its numerous mutants as models has shown that in addition to auxin, ethylene also plays an important role in the regulation of root morphogenesis.4–7 The coniferous Pinus species have a root system consisting of morphologically and anatomically different root types, in which the primary root or the main root has an undetermined capacity for continuous growth, and the lateral roots have a somewhat limited and the short roots a very limited ability, to elongate. The short roots have an important role in nature since they are able to establish ectomycorrhizal symbiosis with several species of mycorrhiza forming fungi mainly belonging to homobasidiomycetes. In ectomycorrhiza the growth of fungal mycelium between the epidermal cells and in the intercellular space of cortical cells affects the meristem so that the short root tip splits to form first a dichotomous root, which through further divisions may form a coralloid root structure.8 The formation of dichotomous roots may also occur without contact with a fungus as a response to stress, and thus it is an endogenous property of the short roots enhanced by some environmental factors.9 The factors regulating the limited growth of the short roots, their ability to host fungal mycelium and the morphological changes at ectomycorrhizal symbiosis have interested the researchers for years.8,10–15 The formation of dichotomous roots has been suggested to result from changes in either auxin or ethylene concentrations due to the fungal growth in the root.15–18
NPA and Short Root Dichotomization
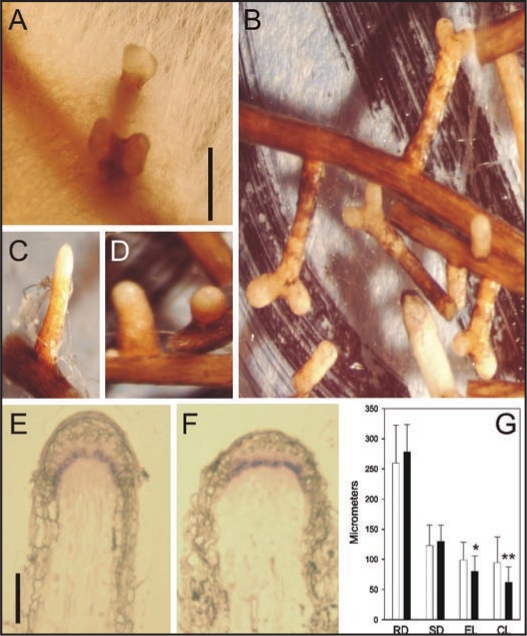
Recently it was confirmed that the polar auxin transport inhibitor N-1-naphthylphthalamic acid (NPA), like mycorrhiza forming fungi, enhances the dichotomization of Pinus sylvestris short roots (Fig. 1A and B). In addition to NPA, precursors of ethylene synthesis13 and ethylene itself17 have been shown to induce dichotomization of short roots comparable to those in mycorrhiza. A similar phenotype is suggested to result from the activation of ethylene biosynthesis by the increased auxin concentration,13 caused by NPA-treatment at the root tip.19,20
Figure 1.
Mycorrhizal and NPA-treated short roots from Pinus sylvestris. Dichotomous mycorrhizal short root tips surrounded by Suillus bovinus mycelium (A). NPA-treated short roots (B). Short roots extending from pieces of lateral roots in control (C) and NPA-treated material (D). In situ hybridisation of Pinus sylvestris SCARECROW (PsySCR) in control (E) and NPA-treated short roots (F). In these longitudinal sections PsySCR transcripts are seen in the short endodermis and at tip between stele and poorly developed root cap. The diameter is often larger in NPA-treated than in control roots although no statistically significant difference was obtained in the measurements (G) for root diameter (RD) or stele diameter (SD). Instead, the length of the endodermis (EL) and root cap (CL) are significantly shorter in NPA-treated than in control roots as seen also in (E) and (F). White and black columns represent control and NPA-treated short roots, respectively. (G) is from ref. 15, Suppl. Material 3. Scale bar in (A) 1 mm and in (E) 100 µm.
The new reports dealing with the hormonal regulation of root growth in Arabidopsis draw attention to the complicated relationship prevailing between ethylene and auxin signaling,4–7 which could also be the acting factor behind the P. sylvestris short root morphogenesis. In comparing the structural similarities and differences in the root structure between Arabidopsis and P. sylvestris, it has to be kept in mind that coniferous roots have an open meristem organization with only one layer of initial cells for cortex, provascular tissue and root cap in contrast to the closed meristem with three layers of initial cells in Arabidopsis roots. Justification for the comparison of the root structure in these species comes from the fact that transcription factor SCARECROW (SCR), which is central in regulating root radial patterning, is expressed in all P. sylvestris root types (see ref. 15, Fig. 1E and F) as in Arabidopsis roots.21
In Arabidopsis analysis of interactions between ethylene and auxin using different ethylene and auxin insensitive and sensitive mutations, ethylene and its precursor treatments, ethylene reporter construct and microarrays have revealed six different gene regulation patterns due to interaction of the two hormones in addition to those being regulated by either auxin or ethylene alone.6 The analysis confirmed also the earlier observations that each hormone could affect the biosynthesis of the other.6,22 Ethylene has also been shown to influence root growth not only through regulating auxin biosynthesis but also by acting on transport-dependent auxin distribution.5 One of the most detailed reports on ethylene regulated processes shows that ethylene increases quiescent center (QC) cell division in A. thaliana roots.4 The new stem and QC cells, however, maintain their functional identity. The latter work shows that ethylene signaling must be tightly controlled to ensure correct cell divisions and cellular organization at root meristem.
Interestingly, both due to the NPA-treatment and contact of short roots with fungal mycelium, the short roots appear to become much broader and shorter than the control short roots (Fig. 1C–F), although it was not possible to show a statistically significant difference in the diameter between control and NPA-treated short roots (Fig. 1G). The organization of the meristem in the swollen NPA-treated short roots also changed (see ref. 15, Fig. 5D and H), which could result from different division patterns in the meristematic cells in NPA-treated than in control short roots. With P. sylvestris short roots embodying open meristem organization it was not possible to perform such a detailed analysis of cell numbers of QC, stem and columella cells as in Arabidopsis roots.4
During dichotomization process two new roots are formed from the swollen root tip in mycorrhizal (Fig. 1A) or NPA-treated (Fig. 1B) short roots. In this process the new tips are located on both sides of a vacuolated central part of the short root and the new endodermis for both root tips differentiates from cells of the provascular tissue in stele (see ref. 15, Fig. 5K–M). The new tips seem to develop from the cells maintaining or acquiring meristematic activity probably due to more moderate accumulation of auxin at the sides than in the center of the apex. In this simplistic model it was further suggested that the accumulation of auxin in the center of the root would induce ethylene biosynthesis, which in turn could induce differentiation of the meristem cells in the apical center of the short roots to vacuolated parenchymatous cells, seen in NPA-treated short roots (see ref. 15, Fig. 5K–M) and reported previously in mycorrhizal dichotomous short roots.8,10,23
PIN Proteins And Auxin Distribution
Auxin efflux transporters, PIN proteins and auxin influx carrier AUX1 localized to specific cell faces have been shown to play an important role in regulating the auxin flow in Arabidopsis root.24–26 The auxin maximum in QC and in columella initial cells results from acropetal auxin transport from the shoot to the root mediated by PIN1 protein located at basal membrane of parenchymatous stele cells. From root apex auxin maximum is distributed to all sides of root cap and further transported by specific PIN proteins upwards in the lateral cap, epidermal and cortical cells for recycling back to the stele.27 PIN proteins encoding genes have also been isolated from other herbaceous and woody plants including hybrid aspen28 and they can also be identified in Pinus taeda and other conifer genomic sequences.29 In the mycorrhizal short roots a tight Hartig net is formed by fungal hyphae in the recycling route, in the apoplastic/intercellular space between epidermal and cortical cells proximal to the meristem. The Hartig net could reduce auxin recycling in the cortex and epidermis24,25 and increase auxin accumulation in the central short root meristem causing the establishment of two lateral apical meristems15 in the same manner as the NPA-treatment is suggested to affect.
Future Aspects
In future research dealing with the root system and ectomycorrhiza development in P. sylvestris must take into account that both auxin and ethylene are involved and consequently, it is not contradictory to obtain the same phenotype with both hormones. Phytohormones are probably also involved in ectomycorrhizal associations in those conifers in which the root morphology is not as dramatically altered as in short roots of pine species.
Although the genomic sequence and transformation system of P. sylvestris are still missing, it has already been shown by genomic resources from other Pinus species (www.tigr.org/tdb/e2k1/pine/pine_status.shtml) that genes like SCR and SHORT ROOT (SHR) regulating root morphogenesis in Arabidopsis occur also in P. sylvestris. The nucleotide sequences of P. taeda and P. sylvestris are highly similar not only in the encoding regions but also in the noncoding regions of the genome (see ref. 15, Suppl. material 1). It is possible that many genes creating auxin gradients and hormonal responses, and known in Arabidopsis may also be identified in conifers due to their highly conserved structure. These genes and their products could be used to learn more about the role of auxin and ethylene in the regulation of growth in the different root types and in the formation of ectomycorrhizal symbiosis in P. sylvestris by using quantitative RT-PCR, in situ hybridization15 and indirect immunofluorescence microscopy.8
The important aspects to test in future root morphogenesis research are: (1) Is the auxin concentration different in main, lateral and short root meristems of pine? (2) Is the retarded growth of short roots caused by low auxin concentration? (3) Does the fungal growth between epidermal and cortical cells of short root affect the basipetal stream of auxin? Furthermore, we should not forget that some ectomycorrhizal fungi themselves are able to produce phytohormone-like compounds which have an influence on root structures.18,30
Addendum to: Cloning of Pinus sylvestris SCARECROW gene and its expression pattern in the pine root system, mycorrhiza and NPA-treated short roots. Laajanen K, Vuorinen I, Salo V, Juuti J, Raudaskoski M. New Phytologist. 2007;175:230–243.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/4972
References
- 1.Baluška F, Šamaj J, Menzel D. Polar transport of auxin: Carrier-mediated flux across the plasma membrane of neurotransmitter-like secretion? Trends in Cell Biol. 2003;13:282–285. doi: 10.1016/s0962-8924(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 2.Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka H, Dhonukshe P, Brewer PB, Friml J. Spatiotemporal asymmetric auxin distribution: A mean to coordinate plant development. Cell Mol Life Sci. 2006;63:2738–2754. doi: 10.1007/s00018-006-6116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortega-Martínez O, Pernas M, Carol RJ, Dolan L. Ethylene modulates stem cell division in the Arabidopsis thaliana root. Science. 2007;27:507–510. doi: 10.1126/science.1143409. [DOI] [PubMed] [Google Scholar]
- 5.Ružicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 2007;19:2197–2212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multiple interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–2285. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibiton of root cell elongation. Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niini S, Raudaskoski M. Growth patterns in non-mycorrhizal and mycorrhizal short roots of Pinus sylvestris. Symbiosis-Rehovot. 1998;25:101–114. [Google Scholar]
- 9.Faye M, Rancillac M, David A. Determinism of the mycorrhizogenic root formation in Pinus pinaster. Sol New Phytol. 1980;87:557–565. [Google Scholar]
- 10.Wilcox HE. Morphological studies of the roots of red pine, Pinus resinosa. II. Fungal colonization of roots and the development of mycorrhizae. Am J Bot. 1968;55:688–700. [Google Scholar]
- 11.Niini SS, Tarkka MT, Raudaskoski M. Tubulin and actin protein patterns in Scots pine (Pinus sylvetris) roots and developing ectomycorrhiza with Suillus bovinus. Physiol Plantarum. 1996;96:186–192. [Google Scholar]
- 12.Tarkka MT, Niini SS, Raudaskoski M. Developmentally regulated proteins during differentiation of root system and ectomycorrhiza in Scots pine (Pinus sylvestris) with Suillus bovinus. Physiol Plantarum. 1998;104:449–455. [Google Scholar]
- 13.Kaska DD, Myllylä R, Cooper JB. Auxin transport inhibitors act through ethylene to regulate dichotomous branching of lateral root meristems in pine. New Phytol. 1999;142:49–58. [Google Scholar]
- 14.Tarkka MT, Nyman TA, Kalkkinen N, Raudaskoski M. Scots pine expresses short-root-specific peroxidases during development. Eur J Biochem. 2001;268:86–92. doi: 10.1046/j.1432-1327.2001.01853.x. [DOI] [PubMed] [Google Scholar]
- 15.Laajanen K, Vuorinen I, Salo V, Juuti J, Raudaskoski M. Cloning of P. sylvestris SCARECROW gene and its expression pattern in the pine root system, mycorrhiza and NPA-treated short roots. New Phytol. 2007;175:230–243. doi: 10.1111/j.1469-8137.2007.02102.x. [DOI] [PubMed] [Google Scholar]
- 16.Slankis V. Hormonal relationship in mycorrhizal development. In: Marks GC, Kozlowski TT, editors. Ectomycorrhizae - Their ecology and physiology. New York, NY, USA: Academic Press; 1973. pp. 232–298. [Google Scholar]
- 17.Rupp LA, Mudge KW. Ethepon and auxin induce mycorrhiza-like changes in the morphology of root organ cultures of mugo pine. Physiol Plant. 1985;64:316–322. [Google Scholar]
- 18.Barker SJ, Tagu D. The roles of auxin and cytokinins in mycorrhizal symbioses. J Plant Growth Regul. 2000;19:144–154. doi: 10.1007/s003440000021. [DOI] [PubMed] [Google Scholar]
- 19.Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 20.Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G, Palme K. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- 21.Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn GM, Feldmann KA, Benfey PN. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86:423–433. doi: 10.1016/s0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 22.Abel S, Nguyen MD, Chow W, Theologis A. ACS4, a primary indolacetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana: Structural characterization, expression in Escherichia coli, and expression characteristics in response to auxin. J Biol Chem. 1995;270:19093–19099. doi: 10.1074/jbc.270.32.19093. [DOI] [PubMed] [Google Scholar]
- 23.Piche Y, Fortin A, Peterson RL, Posluszny U. Ontogeny of dichotomizing apices in mycorrhizal short roots of Pinus strobus. Can J Bot. 1982;60:1523–1528. [Google Scholar]
- 24.Friml J. Auxin transport - Shaping the plant. Curr Opin Plant Biol. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- 25.Leyser O. Auxin distribution and plant pattern formation: How many angels can dance on the point of PIN? Cell. 2005;121:819–822. doi: 10.1016/j.cell.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Petrášek J, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 27.Blilou I, Xu J, Wilwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 28.Schrader J, Baba K, May ST, Palme K, Bennett M, Bhalerao RP, Sandberg G. Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc Natl Acad Sci USA. 2003;100:10096–10101. doi: 10.1073/pnas.1633693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zazímalova E, Krecek P, Skupa P, Hoyerova K, Petrasek J. Polar transport of the plant hormone auxin—The role of PIN-FORMED (PIN) proteins. Cell Mol Life Sci. 2007;64:1621–1637. doi: 10.1007/s00018-007-6566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ditengou FA, Raudaskoski M, Lapeyrie F. Hypaphorine, an indole-3-acetic antagonist delivered by the ectomycorrhizal fungus Pisolithus tinctorius, induces reorganization of actin and the microtubule cytoskeleton in Eucalyptus globulus ssp. bicostata root hairs. Planta. 2003;218:217–225. doi: 10.1007/s00425-003-1095-3. [DOI] [PubMed] [Google Scholar]