Abstract
The cuticle is a physical barrier that prevents water loss and protects against irradiation, xenobiotics and pathogens. This classic textbook statement has recently been revisited and several observations were made showing that this dogma falls short of being universally true. Both transgenic Arabidopsis thaliana lines expressing cell wall-targeted fungal cutinase (so-called CUTE plants) or lipase as well as several A. thaliana mutants with altered cuticular structure remained free of symptoms after an inoculation with Botrytis cinerea. The alterations in cuticular structure lead to the release of fungitoxic substances and changes in gene expression that form a multifactorial defence response. Several models to explain this syndrome are discussed.
Key words: Arabidopsis, innate immunity, Botrytis cinerea, resistance, cuticle
Expression of a Fungal Cutinase Leads to a Strong Immune Response to B. cinerea
Heterologous overexpression of a cell wall-targeted fungal cutinase of Fusarium oxysporum in A. thaliana provides total immunity to B. cinerea.1 The protective effect in these CUTE plants was dependent on the enzymatic activity of the protein, since plants transformed with genes mutated in the active site of the cutinase are no longer protected.1 Plants transformed with lipase A of B. cinerea also exhibit full protection, confirming the importance of the cutinolytic activity for the syndrome observed in CUTE plants.1 Protection is also taking place in plants treated ectopically with cutinase of F. solani. A direct toxic effect against B. cinerea of cutinase or products of the action of cutinase on the cuticle could be excluded1 and the possible involvement of induced plant defences in this response was tested as a first hypothesis. The expression of markers genes PR-1, PR-3, PR-4 and PDF1.2 for the salicylic acid (SA), ethylene (E) or jasmonic acid (JA) pathways were determined. No correlation was observed that might link induced resistance with resistance in CUTE plants.1 The cutinase gene of F. solani was also expressed in A. thaliana mutants of the SA (pad4, sid2), ET (etr1, ein2, pad2) and of the JA (jar1) pathways, but the resistance to B. cinerea observed in CUTE plants is fully independent of SA, ET and JA.1 The idea was further tested if other genes might be linked to resistance in CUTE plants using genome-wide microarrays. Changes in gene expression in B. cinerea-infected CUTE plants were compared to B. cinerea-infected control plants and 15 genes were selected on the basis of an earlier and stronger expression after inoculation with B. cinerea. The expression and accumulation of the products of such genes might explain, at least partially, the full immunity observed in CUTE plants. Constitutive overexpression of each of these genes in separate plants showed that 8 out of 15 genes had a statistically significant effect on the tolerance of the plants to B. cinerea. The genes included three members of the lipid transfer protein (LTP), two members of the peroxidase (PO) and two members of the protein inhibitor (PI) gene families respectively.1 Members of the LTP gene family have previously been shown to be toxic in vitro against various fungi2 and overexpression of LTP from pepper provides resistance to B. cinerea when expressed in A. thaliana.3 POs were proposed to reinforce cell walls most likely by crosslinking lignin monomers4,5 and one putative role for proteinase inhibitors might be to interfere with hydrolytic fungal enzymes involved in the penetration process.6 Tobacco overexpressing a PI gene from Nicotiana alata is better protected against B. cinerea.7 The microarray analysis also revealed changes in gene expression of other genes. For example, changes in the expression of genes encoding for the polygalacturonase-inhibiting proteins AtPGIP1 and AtPGIP2 were also observed. AtPGIP2 is expressed constitutively in CUTE plants, and is induced 36 h after inoculation of WT plants with B. cinerea. Accordingly, extracts of CUTE plants contained a strong PGIP activity in comparison to extracts from WT plants, demonstrating that CUTE plants produce PGIPs prior to infection.8 This is interesting, since PGIPs have been linked to the generation of oligogalacturonide monomers by partially inhibiting the action of fungal polygalacturonase released during the infection process.9 For instance, overexpression of both AtPGIP1 and AtPGIP2 genes in Arabidopsis led to an increase in tolerance to B. cinerea.10 However, overexpression of the CUTE gene in transgenic plants expressing an antisense PGIP did however not lead to increased resistance to B. cinerea.8 Thus, members of the LTPs, PER and PIs might contribute to the resistance induced by B. cinerea in the cuticle altered CUTE plants, while PGIPs might contribute but are not essential for the defence in CUTE plants.
A second hypothesis might be that CUTE plants accumulate and release a fungitoxic metabolite that interferes with fungal growth. The presence of a fungitoxic activity was indeed observed in the inoculation droplets of the B. cinerea spore suspension.1 Gel filtration chromatography and other analyses indicate that the putative substance has a molecular weight smaller than 5000 D, is charged and heat resistant.8 Digestion with protease K reduced the fungitoxic activity of the diffusate.8 At the present the chemical nature of the leaf diffusate remains to be determined.
Mutants Altered in Cuticular Structure Exhibit a Similar Syndrome as CUTE Plants
The intriguing observation made with CUTE plants was completed by a study on a series of mutants impaired in various aspects of cuticle formation. The lcr (lacerata) mutant has a defect in a gene coding for a cytochrome P450 monooxygenase involved in the formation of ω-hydroxy fatty acids in yeast and could be involved in cutin biosynthesis.11 The lacs2 (long-chain acyl-CoA synthetase) also identified as bre112 (Botrytis resistant) mutant has a thinner cuticle than WT plants with a strong reduction in dicarboxylic acid monomers in the cutin polyester.12,13 The ace/hth (adhesion of calyx edges / hothead) mutant, deficient in fatty acid ½-alcohol dehydrogenase activity, shows a reduction in the levels of the major constituents of cuticular polyesters and cutin.14 The bdg (bodyguard) mutant accumulates more cell wall-bound lipids and epicuticular waxes than WT plants.15 Interestingly an increase in resistance to B. cinerea was observed in the lcr, lacs2/bre1 and bdg mutants but not in the ace/hth mutant. In the case of lcr and bdg the resistance correlates with the expression of the same genes as identified in the CUTE plants.8 However, in the case of other mutants showing an increased resistance to Botrytis (such as lacs2/bre1) this correlation was not observed, indicating that other factors must be involved in the resistance to Botrytis.8 A fungitoxic activity could also be observed in the lcr, lacs2/bre1 and bdg mutants.8,12 No fungitoxic diffusate was observed in the ace/hth mutant. The accumulation of a fungitoxic activity could be correlated with the permeability of the cuticle. For instance, the cuticular permeability of CUTE, lcr, lacs2/bre1, bdg8,12 is higher than that of WT controls, whereas no difference was observed between ace/hth mutants and WT plants.12 Interestingly, Tang et al16 also recently observed an association between resistance to Botrytis and increased permeability of mutants in the LACS gene. Thus, the presence of a fungitoxic activity appears to be mostly associated with an increase in cuticular permeability.
The Cuticle: Source of Signals for Defence or Barrier for Diffusion
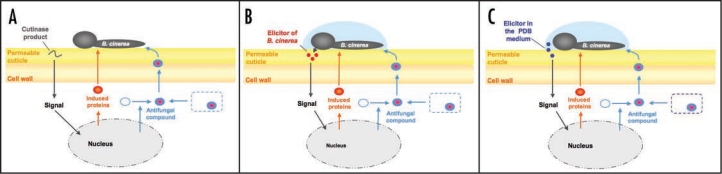
Three scenarios might explain the resistance of plants with defective cuticles (Figs. 1A–C). In the first model (Fig. 1A), the putative products of the cutin monomers released upon the action of the cutinase, will act as intracellular signals and trigger multifactorial defences. Potentially, some of such monomers might also be produced in cuticular mutants as a result of incomplete cuticle formation. These defences might involve the production of antimicrobial proteins and the production or release of antifungal metabolites. In the second model (Fig. 1B), the permeable cuticle of CUTE plants or of cuticle mutants would allow a better uptake of putative elicitors of B. cinerea into the leaf cells. The elicitors might trigger a faster and more intensive defence reaction. Finally, in the third model (Fig. 1C) the elicitors for defence reactions would originate from the PDB (potato dextrose broth) medium used for the inoculation of B. cinerea. The surprising potential for defence against B. cinerea unveiled in CUTE plants warrants further research to understand its molecular basis. Remarkably, A. thaliana is exposed to cutinase and lipase A produced by B. cinerea during infection, yet no resistance is visible. Perhaps the timing or the quantity of enzyme produced is not appropriate for resistance to be induced. Alternatively, B. cinerea, like other pathogens, might suppress induced defence response in the plant.
Firgure 1.
Schematic representations of three scenarios explaining an increased tolerance to B. cinerea. The first model (A) is based on the release of cutin monomers that function as a signal for induced resistance in the plant cell. The response of the plant includes the production of antifungal and other protective proteins as well as the accumulation of an antifungal compound (either by de novo synthesis or release from a compartment). The two other models capitalize on the increased permeability of the cuticle and propose that either elicitors B. cinerea (B) or from the PDB (potato dextrose broth) incubation medium (C) diffuse readily in the cell where they activate the same defences as in (A).
Acknowledgements
The support of the Swiss National Science Foundation (grant 3100A0-104224 to J.P.M. and 3100A0-109405 to C.N.) is gratefully acknowledged.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5071
References
- 1.Chassot C, Nawrath C, Métraux JP. Cuticular defects lead to full immunity to a major plant pathogen. Plant J. 2007;49:972–980. doi: 10.1111/j.1365-313X.2006.03017.x. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Olmedo F, Molina A, Alamillo JM, Rodriguez-Palenzuela P. Plant defence peptides. Biopolymers. 1998;47:479–491. doi: 10.1002/(SICI)1097-0282(1998)47:6<479::AID-BIP6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Jung HW, Kim KD, Hwang BK. Identification of pathogen-responsive regions in the promoter of a pepper lipid transfer protein gene (CALTPI) and the enhanced resistance of the CALTPI transgenic Arabidopsis against pathogen and environmental stresses. Planta. 2005;221:361–373. doi: 10.1007/s00425-004-1461-9. [DOI] [PubMed] [Google Scholar]
- 4.McLusky SR, Bennett MH, Beale MH, Lewis MJ, Gaskin P, Mansfield JW. Cell wall alterations and localized accumulation of feruloyl-3 ‘-methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarisation, peroxidase activity and suppression of flavonoid biosynthesis. Plant J. 1999;17:523–534. [Google Scholar]
- 5.Tognolli M, Penel C, Greppin H, Simon P. Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene. 2002;288:129–138. doi: 10.1016/s0378-1119(02)00465-1. [DOI] [PubMed] [Google Scholar]
- 6.Dunaevskii YE, Tsybina TA, Belyakova GA, Domash VI, Sharpio TP, Zabreiko SA, Belozerskii MA. Proteinase inhibitors as antistress proteins in higher plants. Appl Biochem Microbiol. 2005;41:344–348. [PubMed] [Google Scholar]
- 7.Charity JA, Hughes P, Anderson MA, Bittisnich DJ, Whitecross M, Higgins TJ. Pest and disease protection conferred by expression of barley b-hordothionin and Nicotiana alata proteinase inhibitor genes in transgenic tobacco. Funct Plant Biol. 2005;32:35–44. doi: 10.1071/FP04105. [DOI] [PubMed] [Google Scholar]
- 8.Chassot C. Cuticular defects or wounding lead to full immunity to a major plant pathogen. University of Fribourg; 2006. PhD dissertation. [DOI] [PubMed] [Google Scholar]
- 9.De Lorenzo G, D'Ovidio R, Cervone F. The role of polygalacturonase-inhibiting proteins (PGIPS) in defence against pathogenic fungi. Annu Rev Phytopathol. 2001;39:313–335. doi: 10.1146/annurev.phyto.39.1.313. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 2003;35:193–205. doi: 10.1046/j.1365-313x.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- 11.Wellesen K, Durst F, Pinot F, Benveniste I, Nettesheim K, Wisman E, Steiner-Lange S, Saedler H, Yephremov A. Functional analysis of the LACERATA gene of Arabidopsis provides evidence for different roles of fatty acid w-hydroxylation in development. Proc Natl Acad Sci USA. 2001;98:9694–9699. doi: 10.1073/pnas.171285998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bessire M, Chassot C, Jacquat AC, Humphry M, Borel S, MacDonald-Comber Petétot J, Métraux JP, Nawrath C. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J. 2007;26:2158–2168. doi: 10.1038/sj.emboj.7601658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnurr J, Shockey J, Browse J. The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell. 2004;16:629–642. doi: 10.1105/tpc.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurdyukov S, Faust A, Trenkamp S, Bär S, Franke R, Efremova N, Tietjen K, Schreiber L, Saedler H, Yephremov A. Genetic and biochemical evidence for involvement of HOTHEAD in the synthesis of long-chain α-, ω-dicarboxylic acids in the formation of extracellular matrix. Planta. 2006;224:315–329. doi: 10.1007/s00425-005-0215-7. [DOI] [PubMed] [Google Scholar]
- 15.Kurdyukov S, Faust A, Nawrath C, Bär S, Voisin D, Franke R, Schreiber L, Saedler H, Métraux JP, Yephremov A. The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell. 2006;18:321–339. doi: 10.1105/tpc.105.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang D, Simonich MT, Innes RW. Mutations in LACS2, a long-chain acyl-coenzyme A synthetase, enhance susceptibility to avirulent Pseudomonas syringae but confer resistance to Botrytis cinerea in Arabidopsis. Plant Physiol. 2007;144:1093–1103. doi: 10.1104/pp.106.094318. [DOI] [PMC free article] [PubMed] [Google Scholar]