Abstract
In Arabidopsis, lateral organ initiation correlates with the formation of an auxin maximum in a group of cells at the periphery of the shoot apical meristem (SAM). This signal establishes founder cells that build the lateral organ. Primordia initiation is closely associated with the creation of a functional boundary that separates the newly formed primordium from the remainder of the meristem. In the June issue of Plant Cell, we have characterised the JLO (for Jagged Lateral Organ) gene of Arabidopsis, a member of the Lateral Organ boundary Domain gene family. JLO is expressed in boundaries and regulates both auxin transport, via a negative regulation of PIN auxin export carriers, and meristem fate by promoting the expression of the KNOX genes SHOOTMERISTEMLESS (STM) and BP/KNAT1. In this Addendum, we discuss the regulation of PIN genes by JLO, and propose a model for JLO function during embryonic and post-embryonic development.
Key words: auxin transport, embryo development, meristem, lateral organ, LBD genes
The plant signalling molecule auxin plays a fundamental role in specifying the sites for lateral organ initiation.1,2 The signal that induces protrusion of lateral organs is provided by the appearance of an auxin maximum at the flank of the shoot apical meristem. Polar auxin transport to the meristem is mediated by the coordinated action of influx and efflux carriers. Among the efflux carriers, AtPIN1 represents the main actor of auxin transport in the aerial parts of the plant,3,1 since loss-of-function mutation in the PIN1 gene cause the formation of a needle-like shoot without organs.4
The expression of auxin response genes is regulated through the antagonistic action of auxin response factors (ARF) and auxin response inhibitors (Aux/IAA).5,6 A role for developmental patterning during organ initiation and growth has been assigned to some ARFs such as ARF5/MONOPTEROS (MP) and its inhibitor IAA12/BODENLOS (BDL). Loss-of-functions mutations in MP and dominant mutations in BDL lead to the absence of an embryonic root, the formation of reduced vascular systems and flowerless shoots.7,8 MP may regulate expression of the PIN1 gene,9 but it is so far unknown if MP promotes PIN expression directly or via mediator functions.
PIN1 controlled formation of auxin maxima at the primordia anlagen is tightly correlated with the down-regulation of class 1 KNOX (KNOTTED-Like homeobox) genes, such as STM, BP/KNAT1 and KNAT2 at these sites.10,11 STM is specifically required for SAM formation and maintenance by keeping cells in an undifferentiated state.12,11 STM and PIN1 are expressed in a complementary pattern during organ formation,13 suggesting that local down-regulation of STM may respond to auxin signalling.
In our article,14 we describe a new arrested meristem mutant, called jlo-D for Jagged Lateral Organ. The dominant jlo-D mutant is strongly dwarfed, it forms serrate rosette leaves with short petioles and terminates shoot growth with the formation of a pin-like structure. Our molecular analysis revealed that the gene misexpressed in the jlo-D mutant belongs to the Lateral organ Boundary Domain (LBD) gene family. Proteins of this family are putative transcription factors that carry the conserved LOB domain, consisting of a Zn finger and a leucine zipper-motif.15,16
Formation of an arrested meristem in jlo-D mutants indicates that cells at the flanks of the meristem failed to transit from indeterminate to determinate fates. Consistent with this, we found expanded expression of STM in the terminated meristem. We used inducible misexpression of JLO to identify target genes via Affymetrix microarrays. This study revealed that JLO positively regulates STM and also BP/KNAT1, but represses members of the PIN family (see below). Interestingly, JLO was found to be mainly expressed in the boundary region that isolates the nascent lateral organ from the meristem.
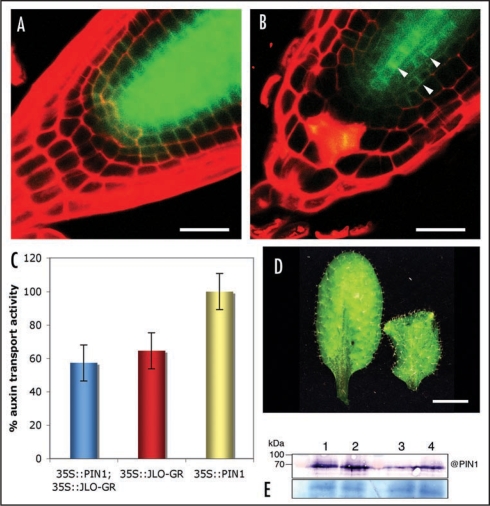
There is a close resemblance between the terminated meristems of jlo-D mutants and those of pin1 mutants,4 suggesting that JLO may interfere with auxin transport. Induced overexpression of JLO caused a reduction of auxin transport to 30% of that measured in wild-type, and decreased expression of the auxin reporter DR5rev: GFP. Supporting our microarray data, we observed decreased PIN1 mRNA levels (down to 15% within 3 hours) correlating with a decrease of PIN1 proteins (Fig. 1) upon JLO induction. Importantly, the polarity of PIN1 proteins is not affected after induction, and residual PIN1-GFP protein is still localized at the plasma membrane (Fig. 1B, arrowhead). However, PIN1 cannot be the only target that is regulated by JLO, because plants expressing PIN1 from the constitutive CaMV 35S promoter show the typical JLO overexpression phenotype after JLO misexpression, and decreased auxin transport (Fig. 1C and D).
Figure 1.
PIN1 expression after JLO misexpression. (A and B) Expression of PIN1:PIN1-GFP in roots of 35S:JLO-GR plants grown on GM medium (A) or GM medium supplemented with 1 µM dexamethazone to induce JLO activity (B). In un-induced plants (A), PIN1-GFP is mainly expressed in vascular tissues and pericycle cells. After induction of JLO expression, PIN1 is limited to the central cells of the stele. Note that PIN1-GFP fusion protein is still membrane localized after induction (arrowheads) of JLO expression and that JLO expression does not appear to alter PIN1 localisation. (C) auxin transport measurement in 35S:PIN1; 35S:JLO-GR (blue), 35S:JLO-GR (red) and 35S:PIN1 (yellow). Note that inhibition of auxin transport is not overcome by an overexpression of PIN1. (D) Leaves of 35S:PIN1;35S:JLO-GR plants before (left) and after (right) JLO induction. Leaves show a JLO overexpressing phenotype, showing that 35S:PIN1 cannot suppress JLO overexpression effects. (E) Top panel: Expression level of PIN1 proteins detected by Western blot using anti-PIN1 antibodies. Lanes 1 and 2: Two independent 35S:PIN1;35S:JLO-GR lines after JLO induction; Lane 3: proteins extracted from wild-type; Lane 4: proteins extracted from 35S:PIN1 overexpressing line. PIN1 protein remains highly expressed in 35S:PIN1;35S:JLO-GR plants. Bottom: Coomassie blue stained loading control. Bars: (A and B) 50 Ém, (D) 1 cm.
Together, our data show that JLO acts upstream of PIN1 and regulates PIN1 expression at the transcriptional level. The suggested role for JLO in regulating auxin export carriers should also be reflected in a loss-of-function phenotype. T-DNA insertion mutants in JLO (jlo-1 or jlo-2) are embryo lethal, and jlo-1 embryos arrest development at the globular stage where they lack provascular cell specification and fail to initiate cotyledons. Thus, JLO is required for normal embryo development. Embryonic apical-basal polarity is specified by local auxin gradients. The partially redundant activities of PIN proteins are central for setting up these patterns, and quadruple PIN mutants (pin1pin3pin4pin7) fail to specify the axis.17 We had found that these 4 PIN genes are also downregulated upon JLO misexpression. We therefore analysed auxin distribution in jlo-1 using a DR5rev:GFP reporter line. In globular stage jlo-1 mutants, auxin accumulates in basal cells of the suspensor, but not in apical cells or the hypophysis, where an auxin maximum is usually observed prior to root meristem specification.17
Several observations strengthen the role of JLO in auxin dependent patterning processes: (1) phenotypes similar to that of jlo-1 were previously described for monopteros (mp) and bodenlos (bdl) mutants; dominant bdl mutations that cause increased stability of the BDL protein and inhibit MP activity cause a lack of auxin accumulation in the hypophysis,9 (2) constitutive expression of MP induces the formation of a needle-like inflorescence shoot18 similar to pin1 and jlo-D mutants; (3) it was recently shown that ARF genes induce expression of several LBD genes that are required for lateral root initiation.19
We propose that JLO expression is activated by ARF proteins that control embryo patterning, such as MP. We noted that the JLO promoter contains several auxin response elements, but where and when this activation takes place remains to be investigated. Differential JLO activation in the embryo may then serve to control and restrict local PIN gene expression, thus permitting a patterned distribution of auxin. During postembryonic development, JLO remains expressed in boundaries, where it serves to activate KNOX gene expression and repress PIN1.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5080
References
- 1.Benkova E, et al. Local efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 2.Reinhardt D, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- 3.Scarpella E, Marcos D, Friml J, Berleth T. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 2006;20:1015–1027. doi: 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vernoux T, Kronenberger J, Grandjean O, Laufs P, Traas J. PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development. 2000;127:5157–5165. doi: 10.1242/dev.127.23.5157. [DOI] [PubMed] [Google Scholar]
- 5.Liscum E, Reed JW. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol Biol. 2002;49:387–400. [PubMed] [Google Scholar]
- 6.Tiwari SB, Wang XJ, Hagen G, Guilfoyle TJ. AUX/IAA proteins are activerepressors, and their stability and activity are modulated by auxin. Plant Cell. 2001;13:2809–2822. doi: 10.1105/tpc.010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Przemeck GK, Mattsson J, Hardtke CS, Sung ZR, Berleth T. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta. 1996;200:229–237. doi: 10.1007/BF00208313. [DOI] [PubMed] [Google Scholar]
- 8.Berleth T, Jürgens G. The role of the monopteros gene in organizing the basal body region of the arabidopsis embryo. Development. 1993;133:575–587. [Google Scholar]
- 9.Weijers D, et al. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev Cell. 2006;10:265–270. doi: 10.1016/j.devcel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Pautot V, et al. KNAT2: Evidence for a link between knotted-like genes and carpel development. Plant Cell. 2001;13:1719–1734. doi: 10.1105/TPC.010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long JA, Moan EI, Medford JI, Barton MK. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature. 1996;379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- 12.Endrizzi K, Moussian B, Haecker A, Levin JZ, Laux T. The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J. 1996;10:967–979. doi: 10.1046/j.1365-313x.1996.10060967.x. [DOI] [PubMed] [Google Scholar]
- 13.Heisler MG, et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol. 2005;15:1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 14.Borghi L, Bureau M, Simon R. Arabidopsis JAGGED LATERAL ORGANS is expressed in boundaries and coordinates KNOX and PIN activity. Plant Cell. 2007;19:1795–1808. doi: 10.1105/tpc.106.047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwakawa H, et al. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for formation of a symmetric flat leaf lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a leucine zipper. Plant Cell Physiol. 2002;43:467–478. doi: 10.1093/pcp/pcf077. [DOI] [PubMed] [Google Scholar]
- 16.Shuai B, Reynaga-Pena CG, Springer PS. The lateral organ boundaries gene defines a novel, plant-specific gene family. Plant Physiol. 2002;129:747–761. doi: 10.1104/pp.010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friml J, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 18.Hardtke CS, et al. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL4. Development. 2004;131:1089–1100. doi: 10.1242/dev.00925. [DOI] [PubMed] [Google Scholar]
- 19.Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell. 2007;19:118–130. doi: 10.1105/tpc.106.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]