Abstract
Microglia engage in the clearance of dead cells or dangerous debris. When neighboring cells are injured, the cells release or leak ATP into extracellular space and microglia rapidly move toward or extend a process to the nucleotides as chemotaxis through P2Y12 receptors. In the meanwhile, microglia express the metabotropic P2Y6 receptors, the activation of which by uridine 5′-diphosphate (UDP) triggers microglial phagocytosis in a concentration-dependent fashion. UDP/UTP was leaked when hippocampal neurons were damaged by kainic acid in vivo and in vitro. Systemic administration of kainic acid in rats resulted in neuronal cell death in the hippocampal CA1 and CA3 regions, where increases in mRNA for P2Y6 receptors in activated microglia. Thus, the P2Y6 receptor is upregulated when neurons are damaged, and would function as a sensor for phagocytosis by sensing diffusible UDP signals.
Key Words: microglia, phagocytosis, P2Y6 receptors, UDP
Accumulating findings indicate that nucleotides play an important role in neuron to glia communication through P2 purinoceptors, even though ATP is recognized primarily to be a source of free energy and nucleotides are key molecules in cells. P2 purinoceptors are divided into two families, ionotropic receptors (P2X) and metabotropic receptors (P2Y) (Fig. 1). P2X receptors (seven types; P2X1-P2X7) contain intrinsic pores that open by binding with ATP. P2Y (eight types; P2Y1,2,4,6 and 11–14) are activated by nucleotides and couple to intracellular second-messenger systems through heteromeric G-proteins.1 Microglia express P2X4, P2X7, P2Y2, P2Y6 and P2Y121 and are known as resident macrophages in CNS, accounting for 5–10% of the total population of glia.2,3 When neurons are injured or dead, microglia are activated, resulting in their interaction with immune cells, active migration to the site of injury, release of pro-inflammatory substances and the phagocytosis of damaged cells or debris. For such activation of microglial motilities, extracellular nucleotides have a central role. Extracellular ATP functions as a chemoattractant. Microglial chemotaxis by ATP via P2Y12 receptors was originally found by Honda et al.,4 and has recently been confirmed in vivo in P2Y12 receptor knockout animals.5 Neuronal injury results in the release or leakage of ATP that appears to be a “find-me” signal from damaged neurons to microglia to cause chemotaxis. In addition to microglial migration by ATP, another nucleotide, UDP, an endogenous agonist of the P2Y6 receptor, greatly activates the motility of microglia and orders microglia to engulf damaged neurons.6
Figure 1.
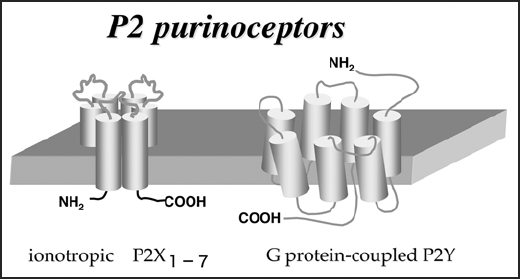
P2 purinergic receptors (ATP receptors).
Phagocytosis is a specialized form of endocytosis taking relatively large particles (> 1.0 µm) into vacuoles and has a central role in tissue remodeling, inflammation and the defense against infectious agents.7 Phagocytosis is initiated by the activation of cell-surface phagocytosis receptors, including Fc receptors, complement receptors, integrins, endotoxin receptors (CD18, CD14), mannose receptors and scavenger receptors8 which are activated by corresponding extracellular ligands called as “eat-me” signals. Since recognition is the most important step for phagocytosis, extensive studies on phagocytosis receptors have been reported. With regard to apoptotic cells, it is well known that dying cells express so called “eat-me” signals such as phosphatidylserine (PS) on their surface membrane,8 by which microglia recognize the apoptotic cells in order to catch and remove them.8 As for amyloid β protein (Aβ), a key molecule that mediates Alzheimer's disease, microglia remove Aβ presumably via Fc receptor-dependent phagocytosis.9,10 It, however, is unclear how phagocytotic cells come to the target cells or debris. Our findings suggest that nucleotides might be the molecules to guide phagocytotic cells to the targets.
We found that exogenously applied UDP caused microglial phagocytosis through P2Y6 in a concentration-dependent manner, and that neuronal injury caused by kainic acid (KA) upregulated P2Y6 receptors in microglia, the KA evoked neuronal injury resulted in an increase in extracellular UTP, which was immediately metabolized into UDP in vivo and in vitro. We also found that UDP leaked from injured neurons caused P2Y6 receptor-dependent phagocytosis in vivo and in vitro. Thus, UDP could be a diffusible molecule that signals the crisis of damaged neurons to microglia, triggering phagocytosis. Nucleotides seem to have the ability to act as “eat-us” signals for necrotic cells suffering traumatic or ischemic injury because such necrotic cells cause swelling, followed by shrinkage, leading to the leakage of cytoplasmic molecules including a large amount of ATP and UTP and extracellular nucleotides are immediately degraded by ecto-nucleotideases, suggesting that leaked nucleotides could be transient and localized signals that alert to the crisis created by the presence of the necrotic cells. These findings suggest that microglia might be attracted by ATP/ADP4,5,11,12 and subsequently recognize UDP, starting to recognize “eat-me” signals attached to the targets and engulf them (Fig. 2). It is interesting that ATP/ADP is not able to efficiently activate P2Y6 receptors, nor can UDP act on P2Y12 receptors. Thus, adenine and uridine nucleotides would regulate microglial motilities, i.e. chemotaxis and phagocytosis, in a coordinated fashion.
Figure 2.
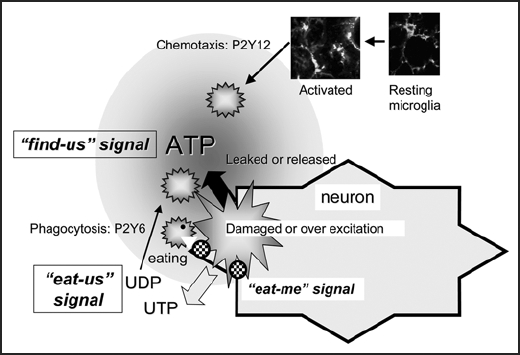
Illustration of nucleotide-activated microglial chemotaxix and phagocytosis. Activated microglia might be attracted by ATP/ADP is not able to efficiently activate P2Y6 receptors, nor ca UDP act on P2Y12 receptors.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/4937
References
- 1.Inoue K. The function of microglia through purinergic receptors: Neuropathic pain and cytokine release. Pharmacol Ther. 2006;109:210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Kreutzberg GW. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 3.Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 4.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tjelle TE, Lovdal T, Berg T. Phagosome dynamics and function. Bioassays. 2000;22:255–263. doi: 10.1002/(SICI)1521-1878(200003)22:3<255::AID-BIES7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 9.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 10.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 11.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 12.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 13.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]


