Abstract
Tubular sprouting in angiogenesis relies on division of labour between endothelial tip cells, leading and guiding the sprout, and their neighboring stalk cells, which divide and form the vascular lumen. We previously learned how the graded extracellular distribution of heparin-binding vascular endothelial growth factor (VEGF)-A orchestrates and balances tip and stalk cell behavior. Recent data now provided insight into the regulation of tip cell numbers, illustrating how delta-like (Dll)4-Notch signalling functions to limit the explorative tip cell behavior induced by VEGF-A. These data also provided a first answer to the question why not all endothelial cells stimulated by VEGF-A turn into tip cells. Here we review this new model and discuss how VEGF-A and Dll4/Notch signalling may interact dynamically at the cellular level to control vascular patterning.
Key Words: Notch, angiogenesis, mouse development, VEGF-A, tip cells
Endothelial tip cells are characterized by their position at the very tip of angiogenic sprouts and by their extensive filopodia protrusions directed towards attractive angiogenic cues.1–9 In the developing mouse retina, tip cells are further distinguished from stalk cells by higher levels of transcripts for certain genes including vascular endothelial growth factor (VEGF) receptor (VEGFR) 2 and near selective expression of other genes like platelet derived growth factor B (pdgf-b).7
Several observations suggest that the tip cell is not a permanent cell fate but rather represents a transient endothelial phenotype that is induced and maintained by VEGF-A stimulation. For instance, exposure of quiescent vessels to VEGF-A leads to new filopodia formation and tip cell gene expression, while VEGF-A sequestration in vivo causes filopodia retraction, suggesting that the tip cell phenotype is reversible. Also conceptually, when sprouts anastomose to form the next vessel loop, the exploratory tip cell behavior must cease to allow for the new connection to stabilize.9,10 We, and others, recently showed that Dll4 signalling through the Notch1 receptor negatively regulates the formation of endothelial tip cells.10–15 Genetic inactivation or pharmacological inhibition of Dll4 or Notch1 signalling leads to excessive formation of tip cells and as a consequence, over-sprouting and a hyper dense vascular network10–15 with immature characteristics.16 Conversely, activation of Notch signalling leads to a reduced number of tip cells and a less dense vascular network.11,12 Sainson et al. first proposed, based on observations in a three-dimensional sprouting system of endothelial cell clusters in collagen gels, that inhibition of Notch signalling leads to increased distal branching through division of tip cells.14 Also, Siekmann and Lawson observed increased proliferation at the tip of sprouting intersegmental vessels in zebrafish after knockdown of RBPJ/suh (required for Notch signalling). This, together with time lapse studies where endothelial cell division was closely monitored, led them to suggest that tip cells undergo stereotyped cycles of sprouting and division.12 However, using clonal analysis of individual cells from RBPJ/suh knockdown embryos, they observed that Notch deficient endothelial cells appeared much more frequently at the tip position while cells with constitutive Notch signalling were excluded from the tip position in an otherwise normal vasculature. Our own mosaic analysis in the mouse retina confirmed that cells deficient in Notch1 more frequently adopt the tip cell position and phenotype. Together, these data allow us to draw important conclusions towards the mechanism of sprouting and the role of Notch signalling. First, individual endothelial cells appear to compete for the leadership. Second, Notch signalling determines which cell will gain competitive advantage among the endothelial cells stimulated by VEGF-A. Third, while Notch may also influence cell division, this effect alone cannot account for tip versus stalk positioning or the dramatic increase in tip cells after Dll4/Notch inhibition during retinal angiogenesis. The observation of single tip cells with RBPJ/suh knockdown leading sprouts in a wild type background zebrafish seems incompatible with the idea of increased tip cell proliferation as the cause of hypersprouting in Notch loss of function mutants. One would otherwise expect to observe clusters of several knockdown cells to out-compete their wild type endothelial cell siblings. However, Lawson cautioned their data did not allow to unequivocally establish whether one or more knockdown cells were situated at the tip of the intersegmental sprouts in the transplant experiments (personal communication). The use of nuclear localized green fluorescent protein (GFP) to mark transplanted cells would help to solve this question in the future. In addition, Lawson commented that loss of Notch signalling in their study affected how many cells decided to proliferate rather than how often individual cell proliferated (or how rapidly they do so). Thus, clearly more work needs to be done to establish whether the increased proliferation contributes to the competitive advantage of Notch deficient cells for the tip position.
How then is competitive advantage achieved? During sprouting angiogenesis in the retina, point sources of VEGF produced by astrocytes act on the tip cells, which express high levels of VEGFR2. Thus, spatial proximity to the point source could favor one cell over the other as higher levels of ligand should lead to higher VEGFR2 signalling activity. However, tip cells also occur in more uniform VEGF environments suggesting that endothelial cells might have to determine their leader in a tug of war. In theory, as a cell that has more VEGFR2 signalling activity should be more likely to adopt the tip position, Dll4/Notch signalling could function to downmodulate this pathway in trailing stalk cells thereby limiting their chances of becoming a tip cell. Indeed, there is good evidence for the cross talk between these two signalling pathways as VEGF-A induces Dll4,17 and Dll4-Notch signalling downregulates VEGFR2 in cultured endothelial cells.18 Suchting and co-workers reported widespread upregulation of VEGFR2 in retinal vessels of early postnatal Dll4+/- mice, leading them to propose that increased tip cell formation from stalk regions correlates with increased VEGFR2 signalling in these regions. Accordingly, the normal function of Notch signalling could be to limit VEGFR2 levels in the stalk in order to prevent ectopic tip cell activity. If such a system was functioning in vivo, it would generate a negative feedback loop, which should indeed be able to select tip and stalk cells even in a uniform VEGF environment (Fig. 1A). In an effort to understand the properties of such a feedback loop and to investigate whether and how tip cell selection would respond to different VEGF-A environment, Bentley and co-workers recently established a computer simulation model of tip cell selection.19 The model illustrates that VEGFR2 regulation downstream of Notch signalling is sufficient to promote stable selection of tip and stalk cells, but only in a narrow window of VEGF-A concentration. Interestingly, the selection process is facilitated through tip cell filopodia extension and a stable pattern is much more rapidly established in non uniform VEGF environments. Several experimental observations suggest that the regulatory mechanism involves additional components of the VEGF signalling pathway. While others have not been able to detect changes in VEGFR2 mRNA levels after Dll4/Notch inhibition,10–12,15 VEGFR1 expression levels were consistently reduced in Dll4+/- retinas and after inhibition of Notch signalling13,15 (and our unpublished observation). Conversely, forced Dll4 expression in cultured endothelial cells leads to increased levels of VEGFR1 and sVEGFR1 (soluble VEGFR1).20 This may be another mechanism induced by Notch signalling to attenuate sprouting activity since VEGFR1 functions as a decoy receptor by limiting VEGFR2 activation (Fig. 1B).21,22 Our own unpublished data further show strong upregulation of neuropilin-1 (Nrp1) following inhibition of Notch signalling. As Nrp1 is considered a positive modulator of VEGFR2 signalling,23 regulation of net VEGFR2 activity, not levels, could be achieved through Notch mediated fine tuning of VEGF co-receptors VEGFR1 and Nrp1. Siekmann and Lawson reported upregulation of yet another receptor for the VEGF family, VEGFR3, in zebrafish following inhibition of Notch signalling.12 VEGFR3 is primarily known for its essential role in lymphangiogenesis where it acts as the key signalling receptor for VEGF-C (reviewed in ref. 24). However, during development, VEGFR3 is also expressed in blood vascular endothelium and important for development of the cardiovascular system.25 Interestingly, also in the developing mouse retina, VEGFR3 is expressed at higher levels in tip cells compared to stalk cells and inhibition of Notch leads to an upregulation of VEGFR3 during retinal angiogenesis (Tammela and Alitalo, personal communication). In line with this, inhibition of VEGFR3 partially rescued normal tip cell patterning after inhibition of Notch signaling in zebrafish12 and mouse retina (Tammela and Alitalo, personal communication). Together, these data reinforce the idea that modulation of VEGF signalling is the key downstream effector of the Dll4/Notch signalling pathway in the process of tip cell selection (Fig. 1C). Selective receptor activation and inactivation, as well as clonal analyses will be necessary to determine the true involvement of the individual receptors and signalling components in the tip cell selection process.
Figure 1.
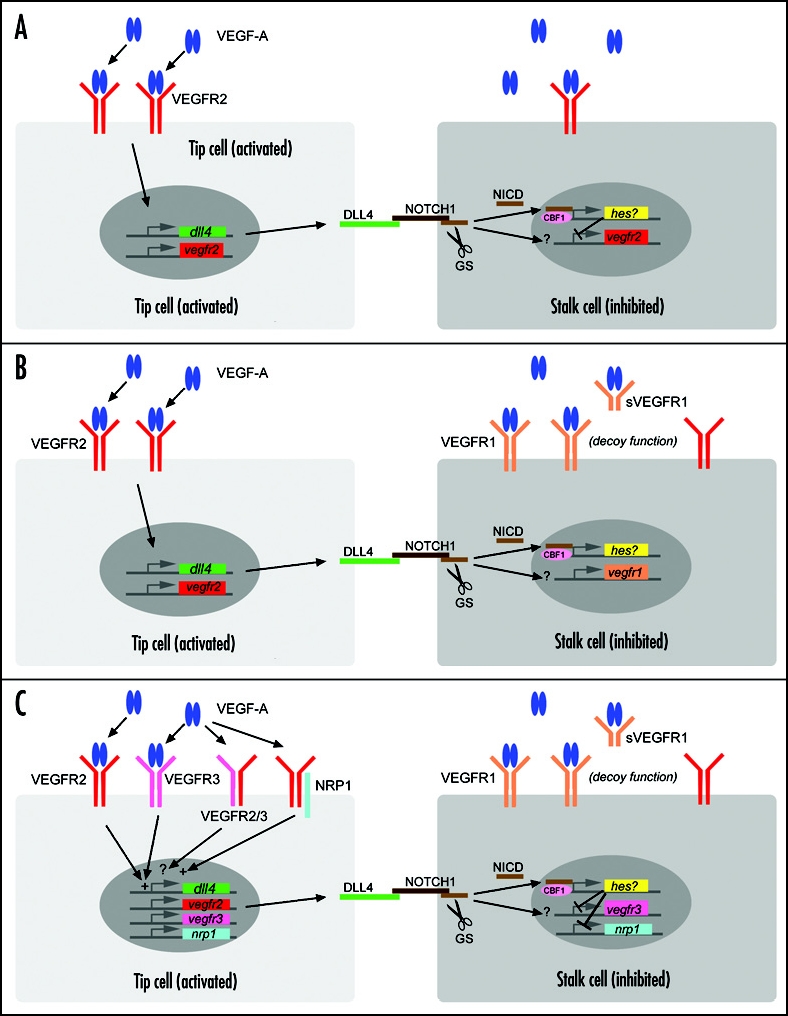
Schematic illustration of possible mechanisms of tip cell selection through modulation of different components of the VEGF signalling pathway following Notch signalling. Two adjacent endothelial cells are depicted, light grey tip cell (activated by VEGF-A), dark grey stalk cell (inhibited through Notch signalling). (A) VEGF-A (blue dimers) binds and activates VEGFR2 in the tip cell, leading to activation of the dll4 promoter and also VEGFR2 promoter reinforcing high levels of VEGFR2 receptor. Dll4 (green) activates the Notch receptor (brown) in the neighboring cell. Gamma secretase (GS) cleavage leads to release and translocation of the Notch intracellular domain (NICD) into the nucleus, where it binds to the transcriptional co-repressor complex including CBF1/RBPJ/suh. NICD binding leads to transcriptional activation of downstream targets of the Hairy enhancer of split (Hes) family. Repression of the VEGFR2 promoter may be mediated by Hes members or possible other direct effects of NICD. Net reduction in VEGFR2 protein in the stalk cell leads to competitive advantage of the tip cell. (B) Additional pathway components are regulated by Notch signalling. VEGFR1 (orange) and soluble VEGFR1 lacking the transmembrane domain sequester VEGF-A in the extracellular space, thus reducing signalling and activity through VEGFR2. NICD through unknown mechanism promotes VEGFR1 promoter activity. (C) VEGFR3 (pink) and neuropilin-1 (NRP1, turquoise) expression is induced in tip cells, promoting net activity of VEGF signalling. Dll4-Notch signalling leads to reduced expression of VEGFR3 and NRP1 in stalk cells. The regulation of VEGFR1 as in (b) augments the net difference in VEGF signalling between tip (high) and stalk (low).
In order to further understand how the Dll4-Notch signalling pathway regulates the sprouting process, we also need to consider the dynamics of Dll4 and Notch protein expression and activation in the context of the endothelial cell behavior. In particular, it will be important to decipher whether the “salt and pepper” pattern of Dll4 expression reflects a spatial and/or a temporal regulation event, and how Notch downstream components distribute and function in a dynamic fashion. dll4 mRNA is most strongly expressed in the sprouting region, but not entirely selective for tip cells. Instead dll4 can be found in both tip and stalk cells, but almost never in directly neighboring cells. This distinct “digital” or “black and white” pattern of Dll4-positive (“white”) and negative (“black”) endothelial cells can only partially be explained by Dll4 induction through VEGF and subsequent Dll4-mediated suppression of VEGFR in neighboring cells, as one would expect to still find some Dll4-expressing cells (“grey”) neighboring each other in such a model where a graded (analogue) VEGF signal is the input.11 Also, while the tip cell marker pdgfb spreads over many neighboring cells when Notch signalling is inhibited, dll4 remains to be distributed in the “salt and pepper” pattern. This would argue that dll4 is not directly regulated through a Notch mediated negative feedback loop. Interestingly, antibody staining for Dll4 showed that the protein was more widely expressed than the dll4 mRNA.11,15 This could suggest that other cells also express dll4 mRNA at very low levels not detected by in situ hybridization and that the Dll4 protein is far more stable compared to the mRNA, thus allowing the protein to accumulate. Alternatively, the in situ hybridisation images could potentially reflect snapshots of high expression, which fluctuates in alternating pulses between neighboring endothelial cells. Interestingly, such pulses or fluctuations in expression are characteristic of the dynamic behavior of Notch pathway components in other developmental systems including vertebrate somitogenesis and neural patterning, reviewed in ref. 26. Although the ligand Dll1 (delta C in zebrafish) oscillates in expression.27,28 the oscillations of Notch signalling are primarily regulated by a negative feedback loop in the Notch downstream targets Hes (Fig. 1), in particular Hes7 and Hes1, as well as lunatic fringe, a glycosyltransferase that modulates Notch intracellular domain (NICD) activity, reviewed in ref. 25. The main function of Notch signalling is not to establish these oscillations, but to synchronize them between the cells in order to achieve regular formation of somite boundaries and axial symmetry. If these cells are segregated, they loose synchronization, but still oscillate individually, however, with unstable periodicity.29 Hes1 oscillations have been observed in many cell types including fibroblasts and neuroblasts. It appears that periodic expression of Notch signalling components is rather the rule than a specialization, but usually they go unnoticed because these oscillations are not in phase (reviewed in ref. 26). It is tempting to speculate that the dynamic pattern of Dll4 expression oscillates in individual endothelial cells, and that we observe the “salt and pepper” pattern because they are out of phase between neighboring cells. Interestingly, similar oscillations in neuroblast differentation and neural tube patterning fulfil two functions, (a) boundary formation and (b) proliferation control (reviewed in ref. 26). Thus, the observed defects in “vascular boundary” formation, i.e., the tip-stalk distinction, and the observed loss of proliferation control after inhibition of Notch signalling in angiogenesis could very well fit with such a model.
A temporal model of such oscillations in angiogenesis would have to suffice to coordinate repetitive cycles of sprouting, branching and fusion. In the retina, the sprouting region advances roughly 250 µm per 24 hours, corresponding to approximately four to five new vascular loops. Thus, the average time it takes from sprout initiation to anastomosis should be approximately six hours. In line with this estimate, Notch inhibition using a γ-secretase inhibitor for three hours only led to increased and more widespread filopodia extension, whereas six hours treatment led to increased branching density in the front row of the advancing vascular plexus.11 Thus, Dll4 mediated signals, if they oscillate, should cycle with a periodicity of six hours. Unfortunately, current Notch reporter tools in the mouse use GFP, which has a half life of more than 12 hours, and preclude therefore detection of the true dynamic pattern of Notch activation in vivo. Indeed, most images of retinas from these reporter mice illustrate several neighboring cells with GFP signal, although often with different signal intensity. This observation supports the idea that signalling is bidirectional and likely more dynamic than what can be resolved with GFP. Also, our finding of internalized localization of Dll4 in neighboring cells during the sprouting process suggested that signalling goes back and forth between endothelial cells at sprouting front.11
Looking ahead we anticipate that the analysis of dynamic Notch signalling in the process of branching (tip cell formation), anastomosis (tip cell fusion), the control of stalk cell quiescence and maturation, and the cross-talk with other pathways will need to be addressed in detail to further increase our understanding of vascular morphogenesis.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/4978
References
- 1.Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43:3500–3510. [PubMed] [Google Scholar]
- 2.Bär T, Wolff JR. The formation of capillary basement membranes during internal vascularization of the rat's cerebral cortex. ZZellforsch. 1972;133:231–248. doi: 10.1007/BF00307145. [DOI] [PubMed] [Google Scholar]
- 3.Marin-Padilla M. Early vascularization of the embryonic cerebral cortex: Golgi and electron microscopic studies. J Comp Neurol. 1985;241:237–249. doi: 10.1002/cne.902410210. [DOI] [PubMed] [Google Scholar]
- 4.Kurz H, Gartner T, Eggli PS, Christ B. First blood vessels in the avian neural tube are formed by a combination of dorsal angioblast immigration and ventral sprouting of endothelial cells. Dev Biol. 1996;173:133–147. doi: 10.1006/dbio.1996.0012. [DOI] [PubMed] [Google Scholar]
- 5.Flamme I, Baranowski A, Risau W. A new model of vasculogenesis and angiogenesis in vitro as compared with vascular growth in the avian area vasculosa. Anat Rec. 1993;237:49–57. doi: 10.1002/ar.1092370106. [DOI] [PubMed] [Google Scholar]
- 6.Flamme I, Frolich T, Risau W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J Cell Physiol. 1997;173:206–210. doi: 10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mato M, Ookawara S. Ultrastructural observation on the tips of growing vascular cords in the rat cerebral cortex. Experientia. 1982;38:499–501. doi: 10.1007/BF01952660. [DOI] [PubMed] [Google Scholar]
- 9.Mato M, Ookawara S, Namiki T. Studies on the vasculogenesis in rat cerebral cortex. Anat Rec. 1989;224:355–364. doi: 10.1002/ar.1092240304. [DOI] [PubMed] [Google Scholar]
- 10.Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- 11.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 12.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behavior in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 13.Suchting S, Freitas C, leNoble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, Hughes CC. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. Faseb J. 2005;19:1027–1029. doi: 10.1096/fj.04-3172fje. [DOI] [PubMed] [Google Scholar]
- 15.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scehnet JS, Jiang W, Kumar SR, Krasnoperov V, Trindade A, Benedito R, Djokovic D, Borges C, Ley EJ, Duarte A, Gill PS. Inhibition of Dll4-mediated signaling induces proliferation of immature vessels and results in poor tissue perfusion. Blood. 2007;109:4753–4760. doi: 10.1182/blood-2006-12-063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: Implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentley K, Gerhardt H, Bates P. Agent-based simulation of notch mediated tip cell selection in angiogenic sprout initialisation. J Theor Biol. 2007 doi: 10.1016/j.jtbi.2007.09.015. in press: http://dx.doi.org/10.1016/j.jtbi.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Harrington LS, Sainson RCA, Williams CK, Taylor JM, Shi W, Li JL, Harris AL. Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvasc Res. 2007 doi: 10.1016/j.mvr.2007.06.006. In press. [DOI] [PubMed] [Google Scholar]
- 21.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 22.Roberts DM, Kearney JB, Johnson JH, Rosenberg MP, Kumar R, Bautch VL. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am J Pathol. 2004;164:1531–1535. doi: 10.1016/S0002-9440(10)63711-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 24.Määkinen T, Norrmen C, Petrova TV. Molecular mechanisms of lymphatic vascular development. Cell Mol Life Sci. 2007;64:1915–1929. doi: 10.1007/s00018-007-7040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 26.Kageyama R, Masamizu Y, Niwa Y. Oscillator mechanism of notch pathway in the segmentation clock. Dev Dyn. 2007;236:1403–1409. doi: 10.1002/dvdy.21114. [DOI] [PubMed] [Google Scholar]
- 27.Maruhashi M, Van De Putte T, Huylebroeck D, Kondoh H, Higashi Y. Involvement of SIP1 in positioning of somite boundaries in the mouse embryo. Dev Dyn. 2005;234:332–338. doi: 10.1002/dvdy.20546. [DOI] [PubMed] [Google Scholar]
- 28.Jiang YJ, Aerne BL, Smithers L, Haddon C, Ish-Horowicz D, Lewis J. Notch signalling and the synchronization of the somite segmentation clock. Nature. 2000;408:475–479. doi: 10.1038/35044091. [DOI] [PubMed] [Google Scholar]
- 29.Masamizu Y, Ohtsuka T, Takashima Y, Nagahara H, Takenaka Y, Yoshikawa K, Okamura H, Kageyama R. Real-time imaging of the somite segmentation clock: Revelation of unstable oscillators in the individual presomitic mesoderm cells. Proc Natl Acad Sci USA. 2006;103:1313–1318. doi: 10.1073/pnas.0508658103. [DOI] [PMC free article] [PubMed] [Google Scholar]


