Abstract
The protein phosphatase 2A (PP2A) family of heterotrimeric serine-threonine phosphatases participates in human cell transformation. Each functional PP2A complex contains one structural A subunit (Aα or Aβ), and mutations of both are found to occur at low frequency in human tumors. We have shown that Aα functions as haploinsufficient tumor suppressor gene by regulating in part phosphatidylinositol 3-kinase (PI3K) signaling. In contrast, loss of Aβ function due to biallelic alterations contributes to cancer progression through dysregulation of small GTPase RalA activity. These observations provide evidence that dysfunction of particular PP2A complexes regulate specific phosphorylation event necessary for cancer initiation.
Key Words: protein phosphatase 2A, RalA, cancer, transformation
Reversible phosphorylation plays a key role in the regulation of signaling pathways relevant to cell transformation. Dysregulation of several kinase oncogenes have been shown to be required for cancer development, and several targeted therapies focused on inhibiting particular kinases have now been approved for clinical use. Although it is clear that phosphorylation is also regulated by phosphatases, initial biochemical studies suggested that unlike kinases, phosphatases act promiscuously and constitutively in vitro. However, recent work indicates that phosphatases play essential roles in malignant transformation by acting on specific substrates in vivo.
Protein phosphatase 2A (PP2A) is a family of serine-threonine phosphatases implicated in the control of a diverse array of cellular processes. The PP2A core enzyme consists of a catalytic C subunit and a structural A subunit. In mammals, two distinct genes encode closely related versions of both the PP2A A and C subunits. The AC dimer recruits a third regulatory B subunit that has been predicted to dictate the substrate specificity and function of the PP2A heterotrimeric complex. Four unrelated families of B subunits have identified to date: B/B55/PR55/PPP2R2, B′/B56/PR61/PPP2R5, B″/PR72/PPP2R3 and Striatin1 (Fig. 1). Recent genetic and proteomic studies implicate clear roles for PP2A subunits in regulating physiological functions and one emerging view is that specific PP2A complexes play critical roles in cell transformation by regulating particular substrates.
Figure 1.
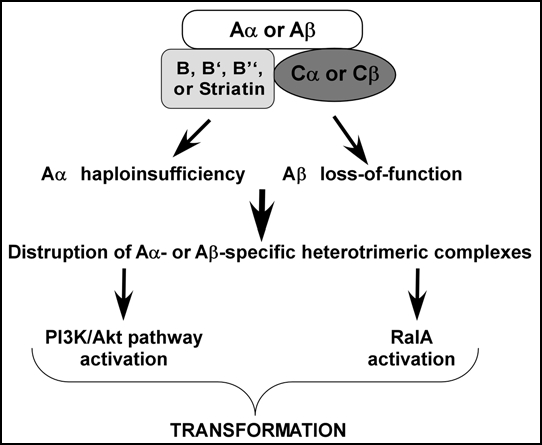
Disruption of PP2A complexes induces transformation. PP2A is a heterotrimeric protein complex, and several isoforms exist for each of the three subunits, creating a diverse family of related enzymes that regulate specific physiological functions. Alterations of PP2A structural subunits, Aα and Aβ, contribute to spontaneously arising human cancers by distinct mechanisms. Cancer-associated Aα haploinsufficiency may induce human cell transformation by activating PI3K/AKT pathway while PP2A Aβ loss-of-function permits the accumulation of activated RalA.
Somatic alterations of the PP2A structural subunit Aβ (PPP2R1B) have been found to occur in colon, lung and breast cancers.2–5 Notably, point mutations in one Aβ allele are commonly accompanied by loss of the second Aβ allele. We confirmed previous work6 that showed cancer-associated Aβ mutants form functionally null alleles.7 These studies indicate that Aβ is genetically inactivated in a subset of human cancers. In addition, we found that suppression of Aβ was found to cooperate with H-Ras, telomerase catalytic subunit hTERT and the SV40 Large T antigen to induce transformation of normal human cells while introduction of wild type Aβ into lung carcinoma cells lacking functional Aβ partially reverses this tumorigenic phenotype.7 Together, these data provide evidence that PP2A Aβ functions as a tumor suppressor gene.
Previous work has shown cancer derived Aβ mutants exhibit markedly impaired ability to form complexes with the catalytic C subunit and the regulatory PR72 subunit.6 We have found that Aβ mutants also showed decreased ability to bind to regulatory Bα subunit and several members of B′ family. These data indicate that cancer-associated alterations of PP2A Aβ result in disruption of most if not all PP2A Aβ-containing complexes. Considering that distinct Aβ-B complexes are likely regulate the phosphorylation of particular substrates involved in transformation, further work is required to identify which B subunits participate in malignant transformation.
Somatic mutations of the more abundant PP2A structural Aα subunit have also been reported in human cancers, although at low frequency.2,8 We previously showed that cancer-associated PP2A Aα mutations contribute to cell transformation by creating a state of haploinsufficiency.9 Although these two distinct PP2A structural isoforms, Aα and Aβ, are 86% identical,10 it was unclear whether these two isoforms share overlapping functions.11 We found that overexpression of Aα failed to revert the tumorigenic phenotype induced by Aβ suppression, suggesting that PP2A complexes containing Aα or Aβ are functionally distinct.
To identify substrates specific for PP2A Aβ, we performed large scale immunopurification of PP2A Aα- and Aβ-containing complexes. We have found that PP2A Aβ complex, but not the PP2A Aα complex, binds to and inhibits activity of the small GTPase RalA through direct dephosphorylation at Ser183 and Ser 194. Cancer-associated Aβ mutants are unable to dephosphorylate RalA, suggesting that loss of Aβ function impairs the formation of complexes with RalA and deregulates its activity. Consistent with previous reports that implicated RalA in regulation of several signaling pathways relevant to cell transformation,12–14 loss of function experiments revealed that RalA is crucial for transformation mediated by Aβ dysfunction. These findings strongly suggest that accumulation of phospho-RalA in PP2A Aβ deficient cells promotes tumorigenic phenotype (Fig. 1). However, we cannot exclude that other substrates of PP2A Aβ complexes also contribute to cell transformation.
These observations also implicate phosphorylation of RalA as an alternative mechanism that may regulate RalA activity and cell transformation. Prior work has shown Aurora A kinase as one kinase that can induce RalA phosphorylation at Ser 194.15 However, further studies are required to identify the kinase(s) that are responsible for RalA phosphorylation at Ser 183 and Ser 194.
While Aβ loss-of-function permits the accumulation of activated RalA, Aα haploinsufficiency seems to induce human cell transformation by activating AKT/PI3K signaling pathway9 (Fig. 1). However, it remains unclear whether PP2A A subunits determine the substrate specificity of heterotrimeric complexes by direct substrate binding, or by forming complex with particular set of B and C subunits. In consonance with the latter idea, Aα and Aβ have been reported to have different affinity to Cα, Bα, B'α1 and PR72 subunits.17 The systematic characterization of PP2A complex composition necessary for RalA dephosphorylation and Akt activation and further structural studies to resolve PP2A in complex with specific substrates will help elucidate the mechanistic details of how PP2A acts as a tumor suppressor.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/4986
References
- 1.Janssens V, Goris J. Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calin GA, di Iasio MG, Caprini E, Vorechovsky I, Natali PG, Sozzi G, Croce CM, Barbanti-Brodano G, Russo G, Negrini M. Low frequency of alterations of the alpha (PPP2R1A) and beta (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene. 2000;19:1191–1195. doi: 10.1038/sj.onc.1203389. [DOI] [PubMed] [Google Scholar]
- 3.Takagi Y, Futamura M, Yamaguchi K, Aoki S, Takahashi T, Saji S. Alterations of the PPP2R1B gene located at 11q23 in human colorectal cancers. Gut. 2000;47:268–271. doi: 10.1136/gut.47.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamaki M, Goi T, Hirono Y, Katayama K, Yamaguchi A. PPP2R1B gene alterations inhibit interaction of PP2A-Abeta and PP2A-C proteins in colorectal cancers. Oncol Rep. 2004;11:655–659. [PubMed] [Google Scholar]
- 5.Wang SS, Esplin ED, Li JL, Huang L, Gazdar A, Minna J, Evans GA. Alterations of the PPP2R1B gene in human lung and colon cancer. Science. 1998;282:284–287. doi: 10.1126/science.282.5387.284. [DOI] [PubMed] [Google Scholar]
- 6.Ruediger R, Pham HT, Walter G. Alterations in protein phosphatase 2A subunit interaction in human carcinomas of the lung and colon with mutations in the A beta subunit gene. Oncogene. 2001;20:1892–1899. doi: 10.1038/sj.onc.1204279. [DOI] [PubMed] [Google Scholar]
- 7.Sablina AA, Chen W, Arroyo JD, Corral L, Hector M, Bulmer SE, Decaprio JA, Hahn WC. The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell. 2007;129:969–982. doi: 10.1016/j.cell.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruediger R, Pham HT, Walter G. Disruption of protein phosphatase 2A subunit interaction in human cancers with mutations in the A alpha subunit gene. Oncogene. 2001;20:10–15. doi: 10.1038/sj.onc.1204059. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Arroyo JD, Timmons JC, Possemato R, Hahn WC. Cancer-associated PP2A Aalpha subunits induce functional haploinsufficiency and tumorigenicity. Cancer Res. 2005;65:8183–8192. doi: 10.1158/0008-5472.CAN-05-1103. [DOI] [PubMed] [Google Scholar]
- 10.Hendrix P, Mayer-Jackel RE, Cron P, Goris J, Hofsteenge J, Merlevede W, Hemmings BA. Structure and expression of a 72-kDa regulatory subunit of protein phosphatase 2A: Evidence for different size forms produced by alternative splicing. J Biol Chem. 1993;268:15267–15276. [PubMed] [Google Scholar]
- 11.Gotz J, Probst A, Mistl C, Nitsch RM, Ehler E. Distinct role of protein phosphatase 2A subunit Calpha in the regulation of E-cadherin and beta-catenin during development. Mech Dev. 2000;93:83–93. doi: 10.1016/s0925-4773(00)00267-7. [DOI] [PubMed] [Google Scholar]
- 12.Camonis JH, White MA. Ral GTPases: Corrupting the exocyst in cancer cells. Trends Cell Biol. 2005;15:327–332. doi: 10.1016/j.tcb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Feig LA. Ral-GTPases: Approaching their 15 minutes of fame. Trends Cell Biol. 2003;13:419–425. doi: 10.1016/s0962-8924(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 14.Feinstein E. Ral-GTPases: Good chances for a long-lasting fame. Oncogene. 2005;24:326–328. doi: 10.1038/sj.onc.1208252. [DOI] [PubMed] [Google Scholar]
- 15.Wu JC, Chen TY, Yu CT, Tsai SJ, Hsu JM, Tang MJ, Chou CK, Lin WJ, Yuan CJ, Huang CY. Identification of V23RalA-Ser194 as a critical mediator for Aurora-A-induced cellular motility and transformation by small pool expression screening. J Biol Chem. 2005;280:9013–9022. doi: 10.1074/jbc.M411068200. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Possemato R, Campbell KT, Plattner CA, Pallas DC, Hahn WC. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell. 2004;5:127–136. doi: 10.1016/s1535-6108(04)00026-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Pham HT, Ruediger R, Walter G. Characterization of the Aalpha and Abeta subunit isoforms of protein phosphatase 2A: Differences in expression, subunit interaction, and evolution. Biochem J. 2003;369:387–398. doi: 10.1042/BJ20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]


