Abstract
Exosomes are small vesicles of endosomal origin that can be released by many different cells to the microenvironment. Exosomes have been shown to participate in the immune system, by mediating antigen presentation. We have recently shown the presence of both mRNA and microRNA in exosomes, specifically in exosomes derived from mast cells. This RNA can be transferred between one mast cell to another, most likely through fusion of the exosome to the recipient cell membrane. The delivered RNA is functional, as the mRNA can lead to translation of new proteins in a recipient cell. The RNA shuttled between cells via exosomes is called esRNA. We propose that several types of exosomes may exist, and that an additional function of exosomes is to communicate to neighbouring cells through delivery of RNA-signals.
Key words: esRNA, exosomes, microRNA, mRNA, cell communication, signalling
Introduction
Exosomes are small membrane vesicles about 30–100 nm in size that are secreted by a variety of cell types such as B-cells, T-cells, mast cells, dendritic cells, platelets, neurons and epithelial cells. The exosome production is initiated when cell membrane proteins transfer to early endosomes by inward budding (Fig. 1). In early endosomes, molecules are either recycled to the plasma membrane or formed to internal vesicles, commonly called multi vesicular bodies (MVBs). In the degradation pathway, the MVBs fuse with lysosomes, but for exosome production, the MVBs fuse with the plasma membrane and consequently release the produced vesicles into the extracellular milieu as exosomes.1 The exosome vesicles consist of a bilayer lipid membrane surrounding a small cytosol which is lacking cellular organelles such as mitochondria, lysosomes, endoplasmic reticulum, nucleus and Golgi apparatus.
Figure 1.
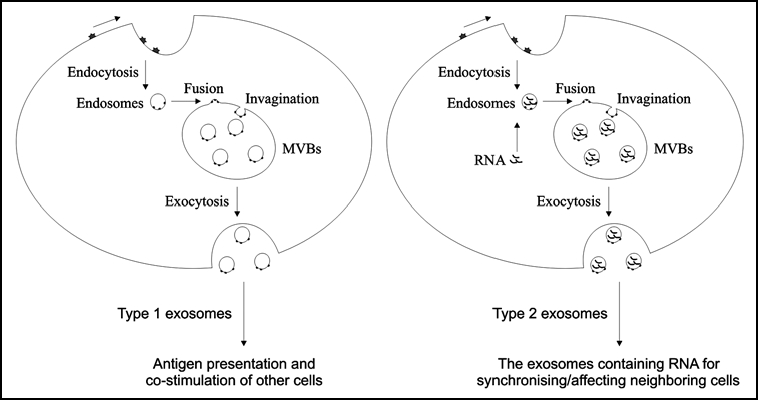
Schematic presentation of different type of exosomes. Type 1 exosomes may be empty of RNA and could be primarily involved in antigen presentation and co-stimulation of other cells. We suggest that the type 2 exosomes contain substantial amounts of RNA, which may be involved in communication/synchronization with neighbouring cells.
Protein Composition of Exosomes
Proteomic analysis from different research groups has proven helpful to understand the nature and role of exosomes, but the protein content of exosomes may vary depending on their source. Co-stimulatory proteins like CD54 (ICAM-1), CD80 and CD86 are commonly present in exosomes derived from antigen presenting cells (APCs). However, exosomes originating from T-cells bear CD3 as specific marker,2 the intestinal epithelial cells express the transmembrane protein A33 on their exosomes3 and neurons enclose subunits of glutamate receptor on their exosomes.4 We have performed an extensive protein analysis of the mast cells line (MC/9) exosomes using FACS analysis, western blot and LC-MS/MS.5 The result showed that the mast cells exosomes contain approximately 270 proteins, many of which previously have been identified in exosomes produced by other cells (i.e., exosomes derived from epithelial cells, urine, dendritic cells, melanoma cells, T-cells and B-cells). The identified exosomal proteins are associated with cell adhesion (CD63), T-cell stimulation (MHCI), translation (elongation factors) as well as protein folding (heat chock proteins). Moreover, a network based analysis of the exosomal proteins shows that these proteins are involved in cell-to-cell signalling, cancer and cellular assembly/organization.
It has been shown that exosomes can mediate antigen presentation in parallel with dendritic cells (DC), B-cells and macrophages. This has lead to the development of exosome-based vaccines as therapy in malignant diseases.6,7 Besides, both the human and mouse tumor cell lines produce exosomes and these vesicles are also present in malignant effusions in patients with different malignancies. The tumor derived exosomes, positive for MHCI, Hsps, CD81 and MART1, can deliver the tumor antigen to DCs followed by activation of tumor-specific T-cells.6,7 The in vivo analysis in mice displayed that the tumor derived exsomes are able to protect against tumor establishment.7
The Putative roles of Exosomal Shuttle RNA
We have shown that exosomes from mast cell line MC/9, bone marrow derived mast cells, and human mast cell line HMC-1, contain substantial amount of RNA,5 whereas no DNA could be found. Using microarray and microRNA chip analysis we have identified 1,300 mRNAs and 120 microRNAs in these mast cell exosomes, many of which seem to be exosome-specific as they were undetectable in the donor cells. Moreover, we have shown that the exosomes carry intact and functional mRNA that can be translated to protein in the presence of functional protein machinery, tested by in vitro analysis, a rabbit lysate in vitro translation assay. Most importantly, our data demonstrate that the mast cell exosomes have the capacity to donate its RNA to another cell, and subsequently affecting the recipient cells protein production. We proved that by adding mouse mast cell exosomes to human mast cells, which lead to delivery of the mouse mRNA to the human cell, and subsequently to the presence of new mouse proteins in the human mast cells. The RNA that can be shuttled between cells via exosomes is suggested to be called “exosomal shuttle” RNA (esRNA).
A network-based analysis of exosomal mRNA predicts functions of esRNA such as “cellular development, protein synthesis and RNA post-transcriptional modification.” This network involved 47 gene products, all encoded by mRNA present in exosomes including transcriptional regulating proteins (HMGN1, MEF2D, RPL7 and SP1), protein kinases (EIF2AK2 and PRKG1), enzymes (ASNS, BRAF, HADHB, PIN4, SOD1 and YWHAZ), translation-regulator protein EIF5A, transmembrane receptor GHR and a number of other products. However, analysis of the exosomal microRNA exhibit number of new functions including stem cell differentiation control (let-7),8,9 differentiation and organogenesis (miR-1),10 hematopoiesis (miR-181)11 and exocytosos and tumorigenesis (miR-17, -18, -19a, -20, 19b-1 and -92-1).12
Although we have documented that mast cells can shuttle RNA between each other, it remains to be investigate how extensively this is occurring in vivo, and how many different cell types have that capacity. Exosomes have been identified in plasma,13 urine,14 malignant effusions15 and bronchoalveolar lavage fluid.16 It is likely that the esRNA is mediating communication in a microenvironment, but the presence of exosomes in serum may suggest that this type of genetic communication theoretically could occur also at a distance. It has been shown that mast cell derived exosomes are able to induce maturation of DCs by upregulation of MHCII, CD40, CD80 and CD8617 or exosomes from antigen presenting cells are involved in T-cell stimulation both in vitro18 and in vivo.19 However, it is not known how much of this communication pathway is dependent on esRNA? It may be that at least two types of exosomes exist, (1) the immunological active exosomes that are involved in antigen presenting and co-stimulation and (2) the type two exosomes that contain RNA and mediating genetic communication between cells. The different types of exosomes may be involved in synchronising a cell type for same function in a certain conditions, or they may influence cells for switching their state from one cell type to another (cellular differentiation). We have shown that the mast cells (MC/9) derived exosomes are able to exchange RNA via exosomes. Theoretically, this exosome mediated communication pathway via exosomes may synchronise surrounding cells to be in the same state as the donor cells, to carry out similar functions in the local microenvironment.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/5114
References
- 1.Fevrier B, Raposo G. Exosomes: Endosomal-derived vesicles shipping extracellular messages. Current Opinion in Cell Biology. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/{zeta} complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 3.Van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 4.Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V. Exosomes are released by cultured cortical neurones. Molecular and Cellular Neuroscience. 31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 6.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 7.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 9.Harfe BD. MicroRNAs in vertebrate development. Curr Opin Genet Dev. 2005;15:410–415. doi: 10.1016/j.gde.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 11.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 12.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 14.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. PNAS. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andre F, Schartz NEC, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T. Malignant effusions and immunogenic tumour-derived exosomes. The Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 16.Admyre C, Grunewald J, Thyberg J, Gripenback S, Tornling G, Eklund A, Scheynius A, Gabrielsson S. Exosomes with major histocompatibility complex class II and costimulatory molecules are present in human BAL fluid. Eur Respir J. 2003;22:578–583. doi: 10.1183/09031936.03.00041703. [DOI] [PubMed] [Google Scholar]
- 17.Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mecheri S. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003;170:3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 18.Raposo G, Nijman H, Stoorvogel W, Liejendekker R, Harding C, Melief C, Geuze H. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, Amigorena S, Thery C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T cell priming. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]


