Abstract
Modern corn (Zea mays L.) varieties have been selected for their ability to maintain productivity in dense plantings. We have tested the possibility that the physiological consequence of the selection involves changes in responsiveness to light and auxin.
Etiolated seedlings of two older corn hybrids 307 and 3306 elongated significantly more than seedlings of a modern corn hybrid 3394. The level of endogenous auxin and activity of PAT in 307 and 3394 were similar. Hybrid 3394 shows resistance to auxin- and light-induced responses at the seedling, cell and molecular levels. Intact 3394 plants exhibited less responsiveness to the inhibitory effect of R, FR and W, auxin, anti-auxin and inhibitors of PAT. In excised mesocotyl tissue 3394 seedlings also showed essentially low responsiveness to NAA. Cells of 3394 were insensitive to auxin- and light-induced hyperpolarization of the plasma membrane. Expression of ABP4 was much less in 3394 than in 307, and in contrast to 307, it was not upregulated by NAA, R and FR. Preliminary analysis of abp mutants suggests that ABPs may be involved in development of leaf angle in corn.
Our results confirm the understanding that auxin interacts with light in the regulation of growth and development of young seedlings and suggest that in corn ABPs may be involved in growth of maize seedlings and development of leaf angle. We hypothesize that ABP4 plays an important role in the auxin- and/or light-induced growth responses. We also hypothesize that in the modern corn hybrid 3394, ABP4 is “mutated,” which may result in the observed 3394 phenotypes, including upright leaves.
Key Words: auxin, auxin-binding protein, growth, leaf angle, light, maize
Introduction
Growth of intact maize seedlings can be regulated by endogenous as well as externally supplied auxin.1–3 Auxin-induced coleoptile growth in corn is controlled by cell elongation whereas the number of cells in the coleoptile remains constant.4 The underlying mechanism of cell elongation remains a subject of debate,5 and two related processes appear to be central. First, auxin activates the plasmamembrane H+-ATPase resulting in membrane hyperpolarization, apoplastic acidification and thus in acid-dependent cell wall loosening, which in turn permits cell expansion (classical acid-growth theory).6–9 Second, auxin increases density of potassium channels in the plasmamembrane and promotes K+ uptake.9,10 On a longer time scale, growth of various organs in intact plants is inhibited by exogenous auxin.11–13 Data of several reports suggest the involvement of anion channels in auxin signal transduction in auxin-dependent growth responses.13–16
Plants growing in the light are shorter than those developing in dark or shade and the inhibition of elongation during photomorphogenesis is nicely evident especially very early after seed germination. The mechanism of light-induced growth inhibition is not yet fully understood, and may include light-induced changes in hormone homeostasis. Various studies have shown correlation between light responses and auxin levels or polar auxin transport17–19,20 (reviewed in ref. 21). An important role of light and polar auxin transport in photomorphogenesis of corn seedlings is deduced from the knowledge of how light-induced changes in auxin transport are involved in the elongation of the mesocotyl. Vanderhoef and Briggs22 and Iino23 reported that red light (R) inhibits mesocotyl elongation and reduces IAA content in the mesocotyl, and the latter is the result of reduction in the supply of IAA from the coleoptile. Later on it was reported that relative to dark, R induces a twofold decrease in free IAA in maize mesocotyl epidermis and rapidly reduces growth rate in epidermal cells.17,24
The interaction of auxin with light may also involve light-induced responses to auxin, e.g., via light-regulated expression of auxin-binding proteins (ABPs). ABP1 has been studied extensively as a putative auxin receptor for growth responses.25,26 Even though a receptor-mediated transcriptional response to auxin has been identified in Arabidopsis recently,27,28 the role of ABP1 in plant growth and development is unquestioned.29–31 Jones et al32 reported that in Arabidopsis R reduces the abundance of ABP1 that controls cell expansion. However, no evidence was provided that ABP1 is involved in auxin-regulated gene expression. In corn, several ABPs have been identified,33 but their functions in growth and development have to be elucidated. It was reported, that, relative to dark-grown plants, reduced elongation of maize seedlings in light correlates with light-reduced expression of ABP1.34
Plants are able to detect neighbor proximity. One of the proposed mechanisms for how plants sense their neighbors, i.e., plant density, is based on the ability of plants to detect changes in R:FR (red: far-red ratio) inside the canopy via R/FR photoreceptors.35–37 Consequently, plants respond to close neighbor proximity by stimulation of elongation growth, reduced branching and a redistribution of leaves to the top of canopy (shade avoidance responses). The molecular mechanisms by which R/FR signals, passed through different phytochromes, are transduced and how they trigger morphological changes in plants are poorly understood. It was proposed that extension growth induced by neighbor detection and shade is the result of R:FR-regulated auxin distribution.38–40
Modern corn varieties have been selected for their ability to maintain production in dense plantings, i.e., where there are likely to be low R:FR conditions. We tested the hypothesis, derived from the work of Morelli and Ruberti40 that the differential sensitivity of a modern (3394) and older (307, 3306) hybrids to crowded conditions may be due to the differential responses to R and/or FR, which in turn affect auxin distribution or sensitivity. This could result in differential morphological changes in the hybrids and, therefore, in their differential ability to respond to neighbor proximity and tolerate plant density. The objective of this study was to examine cross-sensitivity of young 3394 and 307, 3306 seedlings to light and auxin on the whole plant, cellular and molecular level.
Material and Methods
Plant material and growth conditions.
Kernels of three hybrids of Zea mays L., 307 (a double-cross hybrid), 3306 (a single-cross hybrid) and 3394 (a single-cross hybrid) (all from Pioneer Hi Bred, Intl., Des Moines, Iowa; commercially released in the 1930s, 1960s and in the early 1990s, respectively) were used for all experiments. The following abp mutants, i.e., maize (Zea mays L.) plants containing Mutator transposable elements in ABP1 and ABP4 genes,34 were used for analysis of leaf angle development: single mutants abp1 (B2 alleles and B2/K1 alleles), abp4 (B2/K1 and K5 alleles), double mutants abp1/abp4 (B2/K1 and B11/K1 alleles) and WT, a near isogenic line. All mutant seeds were a gift from Alan M. Jones from The University of North Carolina at Chapel Hill.
For sterile (in vitro) cultures, seeds were soaked in 50% (v/v) Reliance solution (Sysco Co., Houston, TX) (∼3% sodium hypochlorite) for 25 min, and then rinsed extensively with sterile distilled water. Seeds germinated on 0.7% (w/v) agar medium in Magenta GA7 boxes (77×77×196 mm) (Sigma, St. Louis, MO) (9 seeds per box). The basal medium (BM) contained Murashige and Skoog salts,41 1% (w/v) sucrose and 1 mM Mes (2-(N-morpholino)-ethanesulfonic acid) (pH adjusted to 6.1 by KOH before autoclaving). The BM was further supplemented or not with auxin (NAA), inhibitors of polar auxin transport (NPA), or with an anti-auxin (PCIB), in various concentrations. Seeds in the Magenta boxes were placed in a growth chamber and incubated at a temperature regime of 23°C. For development of etiolated seedlings, the boxes were wrapped in aluminum foil. Corn seeds were also incubated under either continuous R with maximum irradiance at 660 nm, or continuous FR with spectral irradiance from 720 to 800 nm at 23°C. R was provided by cool white fluorescent tubes (F72 T12CW/VHO, Sylvania, USA) wrapped by red Roscolux filter no. 27 (Rosco, Hollywood Light Inc.). Total photon fluence rate was 13.5 µmol.m−2.s−1. FR light was provided by incandescent bulbs, PS-52 1000 (1000W, Philips Lighting Company, Somerset, NJ) filtered through plexiglass FR filter FRF 700 (Westlake Plastic Company, Lenni, PA). Total photon fluence rate was 53 µmol.m−2.s−1. Fluence rate was measured with a portable spectroradiometer (model LI-1800; Li-Cor; Lincoln, NE) calibrated by the company at the start of experiments.
Measurement of seedling growth and cell length.
The size of various organs was measured with a ruler in 6-, 7- or 11-day-old intact seedlings developed in vitro on BM in various light conditions. Mesocotyl length was measured from scutellar to coleoptilar node, and coleoptile length was measured from coleoptilar node to the tip of the coleptile. Six to nine seedlings per treatment that germinated on the same day were measured in each experiment.
Lengths of epidermal cells were measured on coleoptiles excised from 7-day-old seedlings grown in vitro in dark, R or FR. The coleoptile was excised from the seedling at the coleoptilar node and coated with a thin layer of clear nail polish. After drying, the nail polish image was removed from the coleoptile surface with a transparent tape. Tape with the image was then attached to a microscope slide. Length of cells was measured with a calibrated ocular scale under microscope with a magnification of 100× (Nikon Optiphot, Japan). Four to seven coleoptiles of uniform size were chosen from each treatment and 10 to 20 epidermal cells from basal half of coleoptile (from coleoptilar node to the center of coleoptile) were measured for each coleoptile.
Measurement of leaf angle.
For study of leaf angle development, plants were grown in soil (Sunshine no. 4 soil mix; Sun Gro Horticulture, Bellevue, Wash., USA) in small pots (95 mm high, 105 mm diameter; one seed per pot; 10 mm deep) and regularly watered. Seedlings developed in growth chambers in white light at high R:FR in a 16-h photoperiod and a temperature regime of 23°C-light/21°C-dark. Illumination was provided by cool white fluorescent tubes (F72T12CW/VHO, Sylvania, USA) and incandescent bulbs (100 W, Philips Lighting Company, Somerset, NJ). Total photon fluence rate was 200 µmol.m−2.s−1. Leaf angle (declination from vertical) was measured with a protractor held at the leaf base, as described by Fellner et al.42 in 4 to 6 intact seedlings of each genotype from two to three weeks after seed germination.
Mesocotyl segment growth experiments.
For study of elongation of mesocotyl segments, etiolated seedlings developed in vitro as described above were used as a source of mesocotyl. The 10-mm segments excised just below the coleoptilar node in etiolated 5-d-old seedlings were placed into Petri dishes (60 × 15 mm) containing liquid BM supplemented or not with NAA (0.01 µM to 100 µM). Alternatively, when stated, culture solution containing 1 mM CaCl2, 1 mM MES and 10 mM KCl (pH 6 before autoclaving) was also used. Ten segments were used per genotype and condition in each experiment. Petri dishes were sealed with parafilm and incubated for 24 hrs in the dark or in white light (200 µmol.m−2.s−1), when stated, at 23°C, rotating slowly (50 rpm). After the incubation time, lengths of segments were measured with a ruler to the nearest millimeter and new growth of the segment was calculated. Segments were prepared and measured under green light.
Analysis of endogenous IAA.
For analysis of endogenous free IAA in coleoptiles, 7-day-old corn seedlings grown in vitro in dark, R or FR as described above were used. Whole coleoptiles were excised from the seedling, placed individually into prechilled aluminum foil envelopes, immediately frozen in liquid nitrogen and then stored in a deep-freezer at ∼80°C.
For analysis of endogenous free IAA in corn tissues we followed the method of Chen et al.43 Approximately 0.300 to 1 g of corn coleoptile tissue was ground in a mortar with 4 ml.g−1 of sample in 65% isopropanol with 0.2 M imidazole (Kodak, recrystallized from methanol) buffer 7.0. [13C6]IAA as an internal standard was used at 5 to 10 ng.g−1 coleoptile sample. After extraction for 1 h at room temperature, the extract was centrifuged at 10,000 g for 5 min. The supernatant fluid was then used for analysis of free IAA. Two to 3 ml of supernatant were diluted to 20 to 30 ml, respectively, with double distilled water to reduce the isopropanol concentration, and approximately 30,000 dpm of 3H-IAA (22.5 Ci.mmol−1, Amersham) as a radiotracer were added.
The extract was then applied to a conditioned amino anion exchange column (BAKER-10 SPE 3 ml, Baker, or PrepSep 0.3 g, Fisher). Flow was adjusted to about 5ml/min using a Baker Extraction System. After the diluted extract passed through the column, aspiration was continued for 30 s to remove the excess water, and the column was washed sequentially with hexane, ethyl acetate, acetonitrile and methanol (2.0 ml each). The IAA was eluted from the amino column using 3.0 ml of methanol containing 2% acetic acid. The acidic methanol eluent was evaporated to near dryness using a rotary evaporator, then to dryness using a microrotary evaporator with a two-stage vacuum pump. The residue was resuspended in 100 µl of 50% methanol for HPLC purification. The HPLC procedure was similar to that described earlier,44–46 except that a rapid analytical column (12.5 cm × 4.6 mm) was eluted with 20% acetonitrile/water and 1% acetic acid. The C18 column was packed with 1.2 g of 5 µ Whatman ODS-3 resin using a slurry of 1:1 ethylene glycol:methanol at 8500 psi. The radioactive fractions collected from HPLC were pooled, reduced to dryness, resuspended in 100 µl of methanol, methylated using ethereal diazomethane,47 and analyzed by GC-SIM-MS as previously reported.44
Measurement of membrane potential (Em) of mesocotyl cells.
For these experiments, seedlings were grown in soil in dark at temperature 23°C. Twenty-mm mesocotyl segments excised from upper part of 6-d-old seedlings were used. Seedlings were grown in soil in dark at 23°C. Changes in membrane potential were measured with conventional electrophysiological glass microelectrodes, filled with 300 mM KCl and inserted by micromanipulator into epidermal or cortical cells of the mesocotyl. Tissue was mounted into a perfusion chamber on a microscope stage, which contains the reference electrode. The reference electrode was connected to the bath solution with a liquid junction reference electrode and filled with 300 mM KCl.48 The mounted tissue was continuously perfused with the incubation solution (0.1 mM KCl, 1 mM CaCl2 and 1 mM MES/BTP pH 6.0). After an incubation period of one hour in the dark (in which the mesocotyl cells recovered from excision and regained a sizeable Em) the microelectrode was inserted into the tissue under microscopic control using perpendicular green light of less than 1 µmol m−2 s−1. The microelectrodes were pulled from borosilicate glass capillaries (Kwik-Fil; World Precision Instruments, Saratosa, Fl), backfilled with 300 mM KCl and had tips with resistance ranging between 10 and 30 MΩ, and tip potential of less than 10 mV. After impalement, cells were required to show a steady Em value before various effectors were applied. The effect of white light, or IAA in dark, on membrane potential was tested. White light of 20 µmol m−2 s−1 was applied from a projector through a fiberglass light guide. In the case of the auxin use, at 0 time (auxin ON) the perfusion source was switched to a second reservoir of the same incubation solution plus the IAA (50 µM). The changed solution reached the site of the impalement in 20–30 s. Continuous recordings of the membrane potential of the epidermal cells were made of the first and second cell encountered during impalement. The membrane potential of next deeper cells impaled and assumed to be the cortical cells was recorded as well.
Gene expression analysis.
To compare growth responses of etiolated 307 and 3394 seedlings to light and auxin with expression profiles of maize ABP1 and ABP4 genes, we examined the expression patterns of both genes in the seedlings by reverse transcription PCR (RT-PCR). We studied gene expression in mesocotyls of 5-d-old seedlings grown in D, R or FR, or developed in D in the absence or presence of exogenous auxin.
Total RNA was extracted from the mesocotyl of 5-d-old maize seedlings grown in vitro in dark in the absence or presence of NAA, or under R or FR using RNeasy Plant Mini RNA kit (Qiagen Inc., USA, Valencia, CA) according to the manufacturer's instructions. First-strand cDNA was synthesized from total RNA (1 µg) using Oligo(dT)20 as primer by Thermoscript Reverse Transcriptase (InVitrogen Co., USA, Carlsbad, CA). The cDNA products were directly used in the PCR amplification. PCR reactions of 24 cycles for ABP1 and of 30 cycles for ABP4 were performed by denaturing the template cDNAs at 95°C for 2 min followed by cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 5 min. An ABP1 fragment (maize ABP1 gene accession no. L08425) was amplified using oligonucleotides 5′-CCG CAA AGC AGC TAT GGG ATT-3′ from exon 2 and 5′-CGA AGG GGA ATT TCA GTA CCG CG-3′ from exon 5. An ABP4 fragment (maize ABP4 gene accession no. L08426) was amplified using oligonucleotides 5′-CAG CAG CGC AAC TAC GGG AGG-3′ from exon 2 and 5′-AGT AGG GGA ATT TCA GCT TTG CA-3′ from exon 5. PCR products were size fractionated by electrophoresis in a 1% (w/v) agarose gel stained with ethidium bromide. Expression data were quantified using ImageJ software.
Results
Responses of corn hybrids to exogenous auxin, an inhibitor of polar auxin transport, and an anti-auxin.
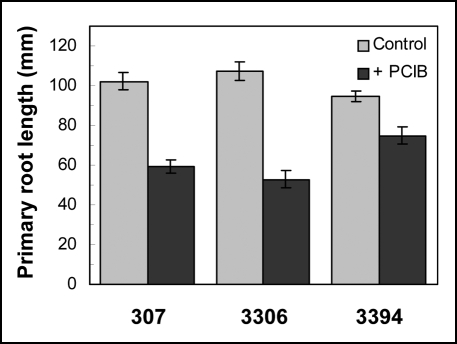
Elongation responses of intact seedlings. In in vitro conditions seeds of three corn genotypes (Zea mays L.) 307, 3306, and 3394 germinated and grew on basal medium (BM). Exogenous NAA added to the BM strongly reduced elongation of primary roots, while the extent of the growth reduction was similar in the new and the older hybrids (data not shown). An inhibitor of polar auxin transport, N-1-naphthylphthalamic acid (NPA), and an anti-auxin p-chlorophenoxyisobutyric acid (PCIB) also distinctly inhibited growth of primary roots in all the hybrids. However, whereas NPA reduced the root elongation to a similar extent in all three hybrids (data not shown), growth of primary roots was reduced by PCIB (50 µM) essentially less in 3394 than in 307 and 3306 (Fig. 1).
Figure 1.
Growth of primary roots in intact etiolated seedlings of older and modern hybrids on the BM supplemented or not with PCIB (50 µM). For each genotype and each condition at least six 7-day-old seedlings were measured in each experiment. Values represent mean ± SE of three independent experiments.
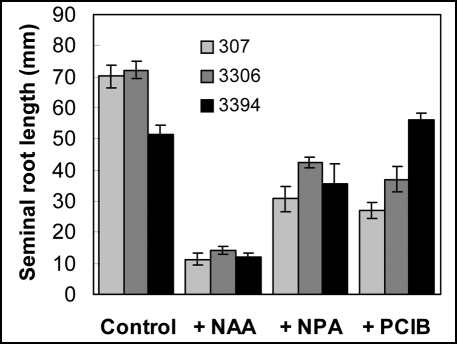
On the level of seminal roots, 3394 exhibited less responsiveness than 307 and 3306 to the inhibitory effect of auxin (50 µM) (Fig. 2). Seminal roots were also distinctly inhibited by NPA (100 µM) and PCIB (50 µM) in 307 and 3306 but negligibly in 3394 (Fig. 2).
Figure 2.
Effect of NAA (50 µM), NPA (100 µM) and PCIB (50 µM) on elongation of seminal roots in intact etiolated seedlings grown in vitro on the BM. For each genotype and condition root length in at least six seedlings was measured in each experiment. For 307 and 3394 values represent mean ± SE of 12 (NAA), 7 (NPA) and three (PCIB) independent experiments. For 3306 values represent mean ± SE of three independent experiments.
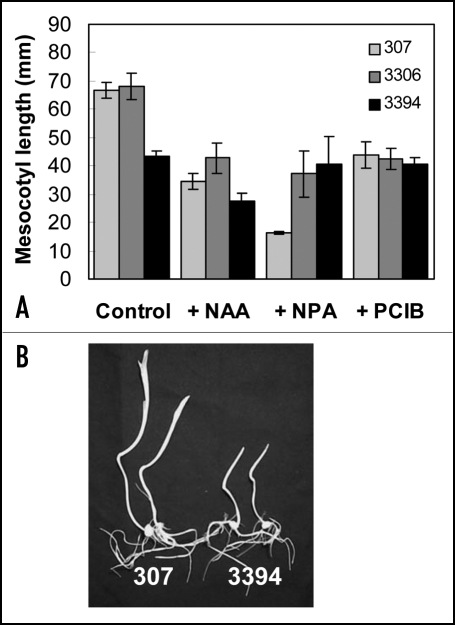
Kinetic experiments revealed that vegetative parts, i.e., mesocotyl and coleoptile, in intact etiolated plants of the three genotypes grown on the BM elongated similarly for the first 2 to 5 days after sowing (data not shown). However, later on seedlings of 3394 grew more slowly than 307 and 3306 seedlings resulting in shorter mesocotyls (Fig. 3A) and coleoptiles in the hybrid (Fig. 3B). As found in roots, the presence of the auxin in the culture medium led to the inhibition of growth in intact etiolated mesocotyls and coleoptiles. NAA at 50 µM strongly inhibited mesocotyl growth in 307 and 3306 (by about 50%), but relatively less in 3394 (by about 30%) (Fig. 3A). At the same auxin concentration coleoptile elongation was inhibited by approx. 45% in 307 and 3306, whereas negligibly in 3394 (data not shown).
Figure 3.
(A) Elongation of mesocotyl in etiolated 7-day old seedlings grown in vitro on the BM supplemented or not with NAA (50 µM), NPA (100 µM) and PCIB (50 µM). For each genotype and condition, root length in at least six seedlings was measured in each experiment. For 307 and 3394 values represent mean ± SE of 12 (NAA), 7 (NPA) and three (PCIB) independent experiments. For 3306 values represent mean ± SE of three independent experiments (for all effectors). (B) Phenotype of etiolated 307 and 3394 seedlings developed on the BM. Seedlings seven days after seed germination are shown.
NPA also reduced growth in etiolated mesocotyl and coleoptile, and the effect was concentration dependent. Figure 3a shows that at 100 µM for example NPA markedly inhibited elongation of etiolated mesocotyl in 307 (by about 70%) and 3306 (by about 50%), whereas it had essentially no inhibitory effect on mesocotyl growth in 3394.
The anti-auxin PCIB at higher concentrations tested, 50 or 100µM, inhibited growth of etiolated mesocotyls by about 35% in the older hybrids, whereas it had no inhibitory effect on mesocotyl elongation in 3394 (Fig. 3A).
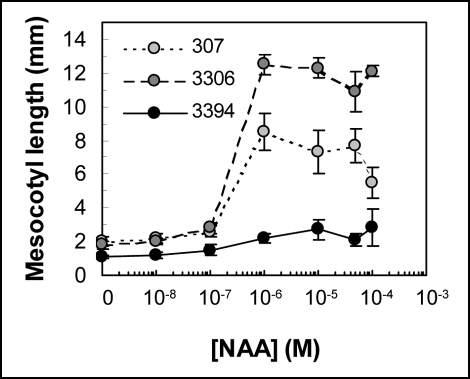
Growth responses of mesocotyl segments to exogenous auxin. In contrast to the responses of intact plants to exogenous auxin, growth of dark-incubated segments excised from etiolated mesocotyls was stimulated by NAA. In 307 and 3306, NAA (1 to 100 µM) promoted elongation of mesocotyl segments with maximum stimulation approx. 300% and 600%, respectively (Fig. 4). As in 307 and 3306, 3394 mesocotyls were most responsive to NAA at a concentration of 1 µM and higher. However, the amplitude of the mesocotyl growth stimulation in 3394 was much less than in 307 and 3306, and reached approx. 100% of stimulation (Fig. 4).
Figure 4.
Growth of corn mesocotyl segments incubated in dark for 24 hrs in liquid BM supplemented or not with NAA. The segments were isolated from etiolated 5-d-old seedlings grown on BM. Initial length of the segments was 10 mm. Data show increase in length (mean ± SE of 20 segments) from one representative experiment. Similar results were observed in three independent experiments for each genotype.
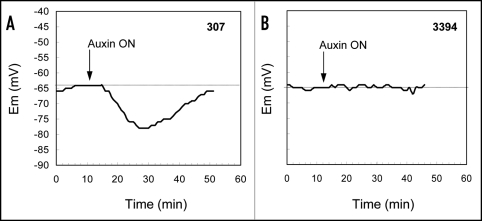
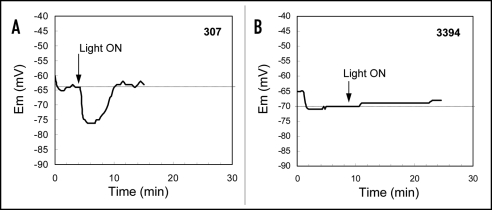
Effect of auxin on membrane potential in etiolated mesocotyls. Measurement of membrane potential (Em) was performed on mesocotyl segments excised from etiolated seedlings of 307 and 3394. After the epidermal or surface cortical cells had been given sufficient time to recover from excision and handling, the perfusion source was switched to a source containing the same solution plus auxin IAA (50 µM). Application of IAA led to distinct hyperpolarization of plasma membrane in 307 epidermal or cortical cells. The hyperpolarization started approx. 4 min after switching the sources (Fig. 5A). The maximum membrane hyperpolarization was reached approx. 20 min after bath solution containing auxin started to flow around the tissue. Afterwards, repolarization of the membrane occurred and was completed after approx. 20 min (Fig. 5A). In contrast to the results for 307, IAA present in the bath solution did not affect membrane potential of cells in etiolated 3394 (Fig. 5B).
Figure 5.
Effect of 50 µM IAA on the Em of corn mesocotyl cortex in etiolated seedlings of 307 and 3394 hybrids. Arrows indicate time when the perfusion source was switched to a second reservoir of the same solution plus IAA. For each genotype and condition data show Em from one representative experiment. Similar results were observed in four independent experiments.
Auxin effect on expression of ABP genes in etiolated corn mesocotyls. Reverse transcription PCR (RT-PCR) indicated that ABP1 was expressed similarly in mesocotyl tissues of the older and modern corn hybrids. Figure 6A, representing an expression profile from an individual experiment, shows that expression of ABP1 in etiolated 307 and 3394 mesocotyl was induced by auxin (NAA) in a concentration-dependent manner even though the auxin effect was very weak. Quantification of the expression data from four independent experiments confirmed that in both genotypes induction of ABP1 expression by auxin was not significantly different from control levels in either genotype (Fig. 6B). On the other hand, higher levels of ABP4 transcripts were detected in auxin-treated 307 plants (Fig. 6A). Unlike in 307, ABP4 expression in etiolated 3394 mesocotyls was very low (Fig. 6B) and the quantification of the results showed that the ABP4 expression in 3394 was not distinctly affected by auxin (Fig. 6C).
Figure 6.
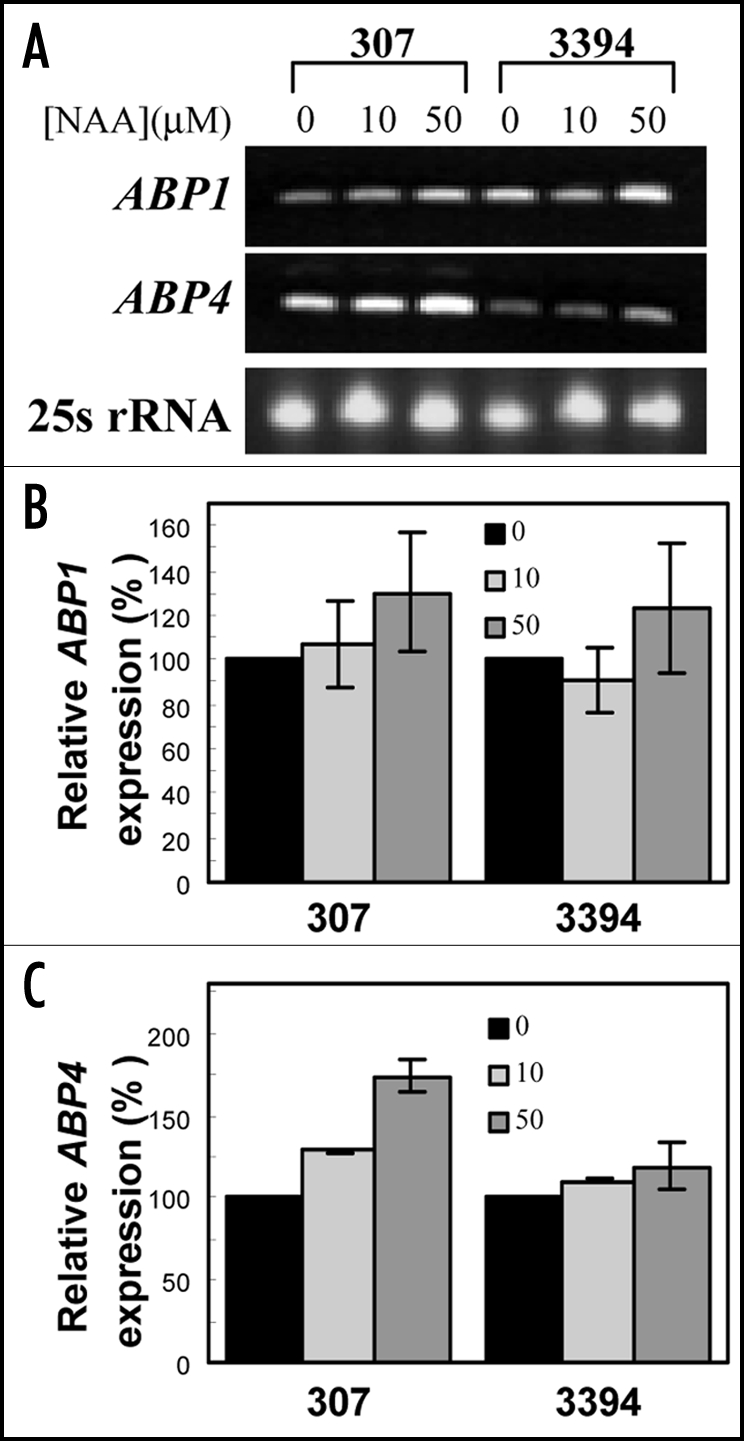
RT-PCR analysis of inducible expression of ABP1 and ABP4 in maize mesocotyls. Etiolated 307 and 3394 seedlings were grown in vitro on BM supplemented or not with NAA. 5-d-old seedlings were used to isolate RNA. One microgram of total RNA was used to synthesize cDNA. The synthesized cDNA was used for amplification of maize ABP1 (403 bp) and ABP4 (403 bp) gene transcripts. In (A) the lowest line is a loading control showing ethidium bromide-stained rRNA. (B and C) show quantification of the expression data (mean ± SE) of four independent experiments for gene ABP1 and ABP4, respectively.
Responses of corn hybrids to light.
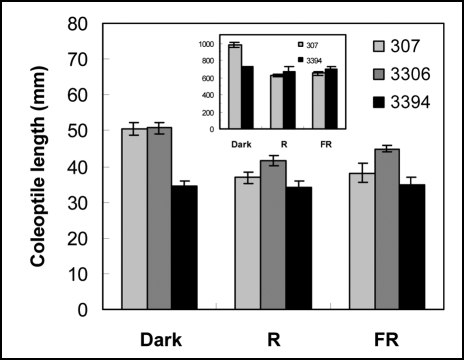
Effect of light on growth of intact seedlings. As mentioned above, in in vitro conditions coleoptiles and mesocotyls of the older hybrids were longer than the organs in the modern hybrid 3394. As in the dark, seeds of all genotypes exposed to R or FR germinated after 2 or 3 days of incubation on the BM. As expected, mesocotyls and coleoptiles of light-developed corn seedlings were shorter than the organs developed in the etiolated seedlings. In accordance with that, R and FR reduced coleoptile length in 7-d-old 307 and 3306 seedlings (Fig. 7). Interestingly, exposure to light had no inhibitory effect on growth of coleoptiles in 3394 seedlings (Fig. 7). Coleoptile elongation was mediated by cell elongation. Epidermal cells in the lower part of etiolated 3394 coleoptiles were shorter than in 307 (Fig. 7, insert; cell length in 3306 was not determined). Also, R or FR reduced the length of epidermal cells of 307 coleoptiles, but not in 3394 (Fig. 7, insert).
Figure 7.
Coleoptile length in 7-d-old seedlings developed on BM in dark, R, or FR. For each genotype and light condition at least six seedlings were measured in each experiment. Values represent mean ± SE of 16 (307 and 3394) and of three (3306) independent experiments. Insert shows length of cells at the basal part of 307 and 3394 coleoptiles developed in D, R or FR. Values show mean ± SE of three independent experiments.
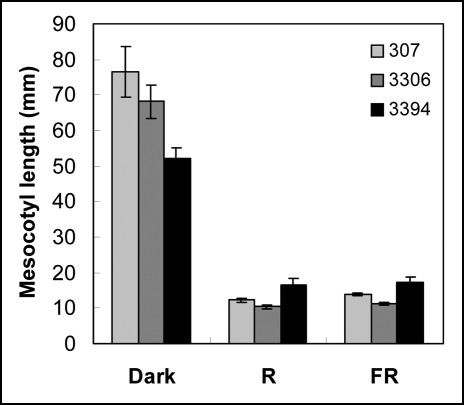
In intact plants light strongly inhibits mesocotyl growth. In the older hybrids 307 and 3306, R and FR inhibited mesocotyl growth by about 80%. Mesocotyl growth of 3394 was less sensitive than 307 and 3306 to the light-induced inhibition and the growth reduction reached about 60% (Fig. 8).
Figure 8.
Mesocotyl length in 7-d-old seedlings developed on BM in dark, R, or FR. For each genotype and light condition at least six seedlings were measured in each experiment. Values represent mean ± SE of 16 (307 and 3394) and of three (3306) independent experiments.
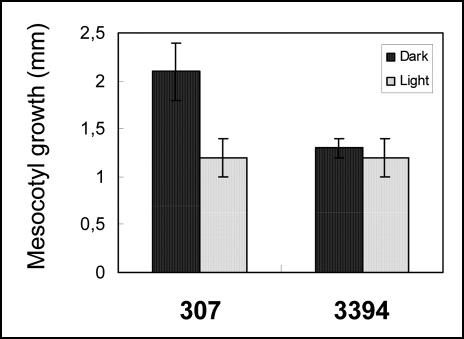
Growth responses of mesocotyl segments to light. Ten-mm segments excised from etiolated mesocotyls of 307 and 3394 were incubated in the presence of 10 mM KCl, 1 mM CaCl2, and 1 mM MES. During 24-hr incubation, 307 segments incubated in dark elongated by 2mm on the average, whereas growth of 3394 segments in dark was almost a half as in 307 (Fig. 9). Interestingly, white light reduced growth by 1 mm during the incubation period in 307, but it had no effect on the growth of 3394 segments (Fig. 9).
Figure 9.
Effect of white light on the elongation of mesocotyl segments incubated for 24 hrs in the presence 10 mM KCl and 1 mM CaCl2. The segments were excised from etiolated 5-d-old seedlings grown in vitro on the BM. Initial length of the segments was 10 mm. Data show increased in length (mean ± SE of 20 segments) from one representative experiment. Similar results were observed two independent experiments.
Effect of light on membrane potential in etiolated mesocotyls. As in the case of auxin, measurement of membrane potential (Em) in response to light was performed on mesocotyl segments excised from etiolated seedlings. After the epidermal or surface cortical cells in 307 genotype had been given sufficient time to recover from excision and handling, the first Em response to white light was rapid hyperpolarization (as seen in Fig. 10A). After 1 to 2 min of illumination, the hyperpolarization reached its peak and membrane repolarization occurred after 2–3 min (Fig. 10A). Approx 5 to 7 min after the illumination started the membrane potential reached the initial level, i.e., Em before light was applied. The response to light occurred much more rapidly than to auxin (see above). As was seen with auxin, light had no effect on Em of cells in etiolated 3394 mesocotyl (Fig. 10B).
Figure 10.
Effect of white light (20 µmol.m−2.s−1) on the Em of corn mesocotyl cortex in etiolated seedlings of 307 and 3394 hybrids. Arrows indicate beginning of white light (20 µmol m−2 s−1) application. For each genotype and condition data show Em from one representative experiment. Similar results were observed in four independent experiments.
Effect of light on expression of ABP genes in etiolated mesocotyls. Reverse transcription PCR indicated that ABP1 was expressed similarly in mesocotyl tissues of seedlings developed in dark (D), R or FR, and that there was no distinct difference in the ABP1 expression between the older and modern corn hybrid (Fig. 11A). Figure 11B shows quantification of the expression data of three independent experiments. In contrast to ABP1, transcription of ABP4 in 307 was distinctly upregulated by R or FR (Fig. 11A and C). In comparison to the older hybrid, the ABP4 transcript in etiolated mesocotyl of 3394 was very low, and importantly it was not significantly affected by light (Fig. 11A and C).
Figure 11.
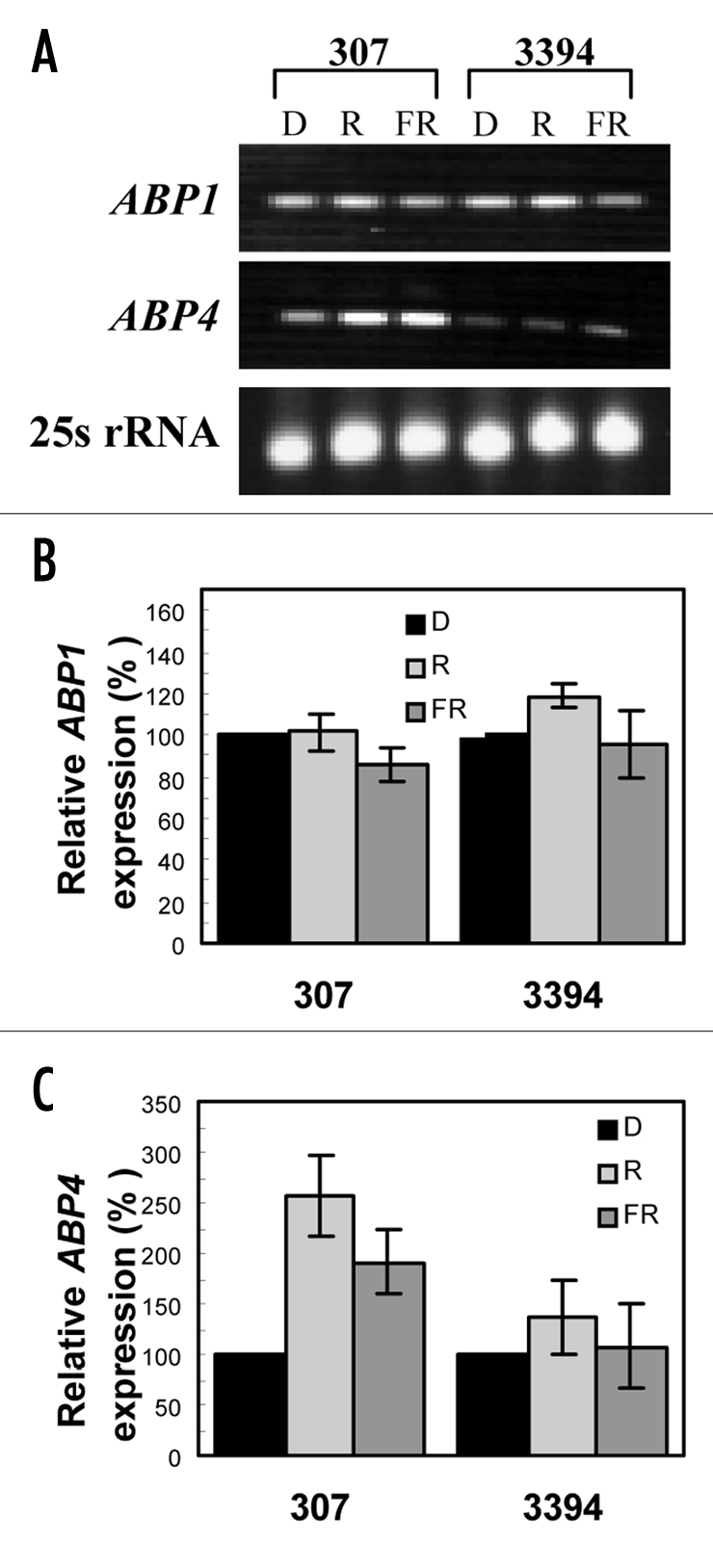
RT-PCR analysis of inducible expression of ABP1 and ABP4 in maize mesocotyls. 307 and 3394 seedlings were grown in vitro on BM in D, R or FR. 5-d-old maize seedlings were used to isolate RNA. One µg of total RNA was used to synthesize cDNA. The synthesized cDNA was used to amplify maize ABP1 (403 bp) and ABP4 (403 bp) gene transcripts. In (A) the lowest line is a loading control showing ethidium bromide-stained rRNA. (B and C) show quantification of the expression data (mean ± SE) of three independent experiments for gene ABP1 and ABP4, respectively.
Cross-talk between auxin and light in plant growth.
A role of light in mesocotyl growth responses to auxin, NPA or PCIB. Exposure to R or FR during germination reduced seedling elongation (Figs. 7 and 8) especially in the mesocotyl. Addition of auxin to R- and FR-grown seedlings had little to no effect on elongation of any seedlings (Table 1, coleoptile results not shown) in contrast to results observed for dark-grown seedlings (Fig. 3A).
Table 1.
Interaction of light, auxin (NAA), an inhibitor of polar auxin transport (NPA) and an antiauxin (PCIB) on mesocotyl elongation in 7-d-old 307 and 3394 seedlings developed in conditions in vitro
| Hybrid | 307 | 3394 | ||||
| Effector | + NAA | + NPA | + PCIB | + NAA | + NPA | + PCIB |
| Conc. | 50 | 100 | 50 | 50 | 100 | 50 |
| Dark | 48 | 70 | 35 | 28 | 12 | 13 |
| R | 0 | 23 | 8 | 13 | 8 | 15 |
| FR | 0 | 26 | 0 | 0 | 11 | 16 |
For each genotype and condition at least six seedlings were measured in each of 3 to 16 independent experiments and mean values were calculated. Values shown in the table indicate percent of inhibition of mesocotyl growth calculated from the mean values.
Similarly, NPA had much less inhibitory effect on mesocotyl elongation in 307 seedlings grown under R or FR, relative to etiolated plants. However, 3394 mesocotyls developed in R or FR responded to NPA similarly as in dark (Table 1).
PCIB inhibited mesocotyl growth by only 8% in R- or had no effect in FR-grown 307 seedlings (Table 1) in contrast to 35% inhibition in etiolated seedlings (Fig. 3A). Mesocotyl growth in R- and FR-grown 3394 seedlings was negligibly inhibited by PCIB, approx. 16% (Table 1), which is similar to the response seen in etiolated seedlings (Fig. 3A).
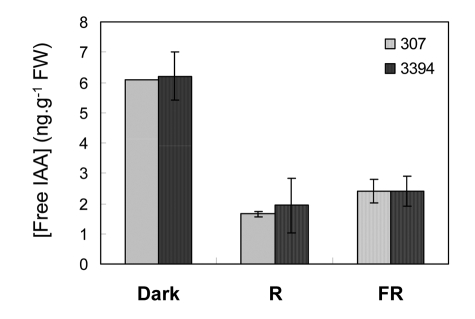
Effects of light on the level of endogenous auxin in corn coleoptiles. Endogenous free IAA was analyzed in 7-day-old coleoptiles in 307 and 3394 seedlings grown in vitro in dark, R, or FR. The two hybrids had similar amounts of free IAA in etiolated coleoptiles, i.e., about 6 ng.g−1 FW (fresh weight) (Fig. 12). In both genotypes, IAA levels in coleoptiles developed under R or FR were about three times lower than in dark-grown seedlings (Fig. 12).
Figure 12.
Level of free IAA in coleoptiles of 7-d-old seedlings of 307 and 3394 hybrid developed in vitro in dark, R, or FR. Auxin analysis was performed by gas chromatography selective ion-monitoring mass spectrometry. Values represent mean ± SE of two independent experiments.
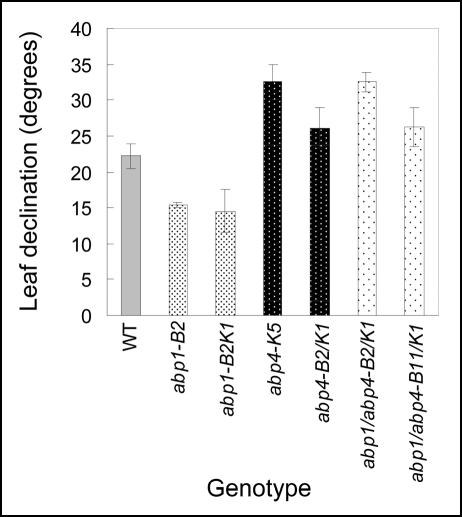
Development of leaf angle in abp mutants.
In 19-day-old WT plants the declination of the 2nd leaf blade from vertical was about 23° (Fig. 13). Leaf angle in abp1 mutants (both alleles) was significantly less than in WT and reached approx. 15°. Both abp4 mutants developed leaves at declinations almost twice as big as abp1, i.e., between 25 to 30° (Fig. 13). Interestingly, both double mutants abp1/abp4 exhibited leaf declination similar to that of abp4 mutant (Fig. 13). Similar results were observed for 3rd and 4th leaves in 21-day-old mutant plants (data not shown).
Figure 13.
Leaf angle of single mutants abp1, abp4, double mutants abp1/abp4 and corresponding WT grown under white light. Leaf angle, measured as a declination from vertical in 19-day-old plants, was determined by a protractor. For each genotype four to six seedlings were measured in each experiment. Values show leaf angle (mean ± SE) obtained in one representative experiment. Similar results were observed in two independent experiments.
Discussion
Role of auxin in growth of etiolated corn seedlings.
Plant growth and development are controlled by numerous external and internal factors. For example, light is one of the most important external factors, and phytohormones are major endogenous regulators. Among the plant hormones, auxin has an important role in cell division, cell elongation, apical dominance and vascular development. Although auxin is believed to stimulate elongation growth in many systems, exogenous auxin applied to intact Arabidopsis plants inhibits hypocotyl growth.13 The functions of auxin in growth strongly depend on its controlled polar transport, making auxin unique among plant hormones. This is supported by many observations that the reduction of basipetal polar auxin transport by synthetic inhibitors, such as NPA, results in alteration (mostly inhibition) of plant growth.49,50
In our study, NPA strongly inhibited growth of intact etiolated seedlings of the older maize hybrids 307 and 3306 and caused coleoptile and mesocotyl agravitropism. This indicates that active basipetal auxin transport is important for elongation in dark-grown corn seedlings. Our results confirm reports that auxin transported in a polar manner is positively involved in growth of etiolated corn seedlings.51–55 It has been reported in Arabidopsis that NPA has little effect on hypocotyl elongation in dark-grown seedlings,56,57 and that polar auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis.19 This suggests that different mechanisms play a role in elongation of etiolated seedlings in Arabidopsis versus corn.
Our growth study on etiolated corn seedlings in vitro showed that, relative to the older hybrids 307 and 3306, there is a block in 3394, causing reduction of elongation growth over the entire young etiolated seedling (Fig. 3B). In addition, a characteristic feature of etiolated 3394 seedlings was reduced responsiveness or resistance to the inhibitory effects of NPA. In fact, the inhibitor applied to etiolated 307 and 3306 seedlings phenocopied elongation in etiolated control 3394 plants. The results suggest that in comparison with the older hybrids, the reduced capacity of etiolated 3394 seedlings to elongate may result from a reduced capacity of polar auxin transport. However, in etiolated seedlings the intensity of PAT42 as well as its velocity (data not shown) were similar in older and modern corn hybrids. This suggests that elongation of etiolated seedlings does not reflect the activity of PAT. Interestingly, elongation of etiolated maize seedlings including seminal roots of the modern hybrid 3394 was also distinctly less inhibited by exogenous auxin. Thus, the resistance of 3394 to the inhibitors of PAT may reflect reduced ability of 3394 to respond to auxin.
In order to determine whether resistance of 3394 to NPA might result from reduced responsiveness to auxin, we tested the effect of PCIB on growth of etiolated seedlings. PCIB is an anti-auxin, or auxin-antagonist, likely competing with auxins for their receptors.58–62 Our results show that, depending on its concentration, PCIB altered elongation of 307 and 3306. This suggests that PCIB applied alone interfered with the effect of endogenous auxin in elongation growth. PCIB also distinctly interfered with the effect of NAA, while the auxin effect strongly depended on PCIB concentration (data not shown). Consistent with results of Foster et al,58 the inhibitory effect of auxin on growth in etiolated seedlings can only be overcome by certain optimal concentrations of PCIB. Very importantly, PCIB had no significant effect on elongation of 3394 (Figs. 1–3). This strongly suggests that, relative to 307 and 3306, the responsiveness to auxin may be reduced in etiolated 3394 seedlings. This conclusion was supported by the fact that, although coleoptiles in 3394 are much shorter than in 307, the level of endogenous free IAA in intact etiolated coleoptiles is similar in both genotypes. It was reported that PCIB itself can inhibit polar auxin transport.63–65 However, since the capacity of PAT was similar in the modern and older hybrids, we could possibly exclude differential inhibitory effects of PCIB on PAT in the hybrid seedlings.
In another experimental system, growth of etiolated segments in the absence of exogenous auxin was basically less in 3394 than in 307 and 3306. In this excised tissue system, exogenous auxin stimulated growth, and we found that NAA promoted elongation of mesocotyl segments much less in 3394 than in 307 and 3306. Importantly, the dose-response curves for the older hybrids and 3394 peaked at a similar concentration range, but they differed in their slope. This suggests that the hybrids do not differ in the affinity of receptors (affinity), but rather in their number, i.e., receptivity.66 Namely, fewer auxin receptors could be present in target tissues of etiolated 3394 seedlings than of 307 or 3306 plants.
Perfusion of IAA solution through segments of various plants, including maize, induces a membrane hyperpolarization that is followed by an acceleration of elongation growth (reviewed in ref. 9 and 67). In agreement with this, in our experiments IAA at 10 µM caused a pronounced membrane hyperpolarization in etiolated mesocotyl cells of 307. This is in accord with conspicuous auxin-induced growth of etiolated 307 segments. Very interestingly, etiolated mesocotyl cells in 3394 were resistant to auxin-induced membrane hyperpolarization, corresponding to negligible elongation of the etiolated 3394 segments in the presence of NAA. In summary, 307 was distinctly sensitive in all auxin-induced responses tested: in responsiveness of intact plants and segments to exogenous auxin, inhibitor of PAT, anti-auxin, and in membrane hyperpolarization.
Study of the expression of genes coding for maize auxin-binding proteins (ABPs) produced very interesting results. We showed that in etiolated mesocotyls and in the absence of exogenous auxin, there was significantly lower expression of ABP4 in 3394 in comparison with the gene expression in 307. In the same conditions, there was no distinct difference between 3394 and 307 in the expression of ABP1. Thus, reduced growth of etiolated 3394 seedlings in the absence of exogenous auxin correlates with very low expression of ABP4 in 3394. Moreover, in etiolated 307 plants expression of ABP4 was upregulated by exogenous auxin in a concentration-dependent manner. This correlates with increased responsiveness of 307 to auxin in cellular as well as in growth responses. On the other hand, the resistance to auxin in etiolated 3394 seedlings for cellular and various growth responses was associated with auxin-insensitive expression of ABP4.
Interaction of light with auxin in elongation growth.
Elongation growth in etiolated seedlings of older hybrids 307 and 3306 was significantly more responsive than growth in 3394 plants to the inhibitory effect of red and far-red light. This was observed on the level of intact plants, segments as well as on the cellular level. The effect of light on seedling growth may be mediated by auxin and/or polar auxin transport.3,17,24,32,51–54 Interestingly, R and FR reduced the level of endogenous IAA and intensity of PAT to the same extent in the older and modern hybrids (Fig. 12; ref. 42). Also, light had no significant effect on velocity of PAT in either genotype (data not shown). This suggests that a factor other than the light-regulated level of endogenous auxin and PAT contributes to the light-induced inhibition of seedlings growth. Seedlings of older hybrids grown in R or FR lost much of their responsiveness to exogenous auxin, NPA and PCIB in comparison with etiolated seedlings (Table 1). This was much less evident in 3394. In fact, seedlings of modern corn hybrids grown in the light responded to NPA and PCIB just like etiolated plants. This suggests that light may reduce seedling receptivity to auxin, which may control growth of corn seedlings in light. This idea was supported by results at the molecular level.
In both hybrids, expression of the ABP1 gene coding for a putative auxin receptor68–70 was not responsive to light. However, in intact 307 plants the reduction of elongation growth by light was associated with light-induced expression of ABP4, relative to the low expression of ABP4 in etiolated plants. Interestingly, lack of R- and FR-induced expression of ABP4 in 3394 seedlings was associated with the hybrid's resistance to R- and FR-induced inhibition of seedling elongation. Our results are consistent with the observation of Im et al.,34 who found that in etiolated corn seedlings ABP1 expression was higher than expression of ABP4, and that ABP4 was upregulated in light-grown seedlings.
Our results suggest that in corn seedlings ABP4 may play a role in auxin- and light-induced growth responses. Since ABP4 can be regulated by auxin as well as by light, its role could be to integrate auxin and light signaling pathways involved in maize growth and development. The association of auxin- and light-induced overexpression of ABP4 with auxin- and light-induced inhibition of elongation led us further to hypothesize that ABP4 may function as a negative regulator of elongation growth of corn seedlings. However, the fact that in the absence of exogenous auxin low expression of ABP4 in etiolated 3394 plants is associated with shorter stature of etiolated 3394 seedlings indicates, that ABP4 would negatively control a positive regulator of elongation growth. Im et al34 made an interesting observation. Using loss-of-function mutants in maize ABP1 and ABP4 genes, the authors found that whereas the abp4 mutant lacks completely the ABP4 transcript, ABP1 level in abp4 mutants was 4–7 times higher than in the corresponding WT, while the level of ABP1 transcript was the same in abp4 and WT. They therefore concluded that elimination of the ABP4 gene activates ABP1 expression post-transcriptionally.34 These results may support our hypothesis. We hypothesize that in the absence of exogenous auxin, low expression of ABP4 in etiolated 307 seedlings (relative to light-grown plants) would result in a high amount of ABP1, which could be a positive regulator of elongation, and thus could lead to elongation of etiolated seedlings. In light, or in dark but in the presence of exogenous auxin, ABP4 is overexpressed in 307. This would reduce the level of ABP1, which would result in less elongation, i.e., in inhibition of elongation. In the modern corn hybrid 3394, ABP4 may be “mutated,” which would result in its basal minimum transcription (Fig. 6A). A basal level of ABP4 could prevent accumulation of ABP1, causing the reduced growth of etiolated seedlings, and their reduced responsiveness to light and auxin in 3394. In contrast to the observation of Im et al.,34 who reported no phenotypic differences between the mutants and WT, we found distinct differences between WT and abp mutants in leaf angle development (Fig. 13). Our results along with data of Im et al.34 suggest that increasing leaf declination (i.e., increased auricle growth, ref. 42) is positively associated with lack of ABP4 transcript or with abundance of ABP1. Namely, ABP1 amount is in abp1 < WT < apb434 and similarly leaf angle is in abp1 < WT < apb4 (Fig. 13). In contrast to the abp4 mutant, which completely lacks ABP4 transcript and develops leaves with large declination, the modern hybrid 3394 developing upright leaves retains a basal level of ABP4 transcript. We have not yet analyzed the level of ABP1 product in 3394. However, we speculate that the minimum functionality of ABP4 in 3394 reduces the accumulation of APB1. It may therefore result in a lower level of ABP1, relative to hybrid 307, and lead to the development of erect leaves in comparison with 307. While abp1/abp4 mutant lacks ABP1 and ABP4 products, leaf declination of the double mutant is similar to that in the abp4 mutant. A possible explanation could be that another member of ABP gene family33 may participate on development of leaf angle in the double mutant.
In the ongoing project supported by the Ministry of Education of the Czech Republic (no. 1P05ME792, to M.F., 2005–2008) we believe to provide evidence, which would test the hypotheses mentioned above.
Acknowledgements
We thank Doug Ewing for maintenance of experimental corn plants in the greenhouse and for technical help. We also thank Jerry Cohen (University of Minnesota) for the opportunity to perform auxin analysis in his lab, and the people in his lab for technical help. We further thank Kari Stiles and Rainer Stahlberg for technical help with the electrophysiological measurements, and students of University of Washington, Nick Stephens, Jaime Forbes, Ragina Smith, and Claire McWilliams of Franklin High School for technical help with growth experiments. We thank Ben Hall for opportunity to perform gene expression experiments in his lab, and Jie Luo from his lab for his advice, technical help with the experiments, and valuable discussion of the results. We also thank Luca Comai for the opportunity to use rooms for experiments with radioactive material. We further thank Luca Comai and Andreas Madlung in his lab, for advice on gene expression experiments. We also thank Alan M. Jones (University of North Carolina) for providing the maize abp mutant seeds. Last, but not least we thank Bob Cleland for his advice during various experiments and for critical comments on the manuscript. This work was supported by Pioneer Hi-Bred Intl., Johnston, Iowa.
Abbreviations
- ABP
auxin-binding protein
- BM
basal medium
- D
dark
- Em
membrane potential
- FR
far-red light
- NAA
1-naphthalene acetic acid
- NPA
N-1-naphthylphthalamic acid
- PAT
polar auxin transport
- PCIB
p-chlorophenoxyisobutyric acid
- R
red light
- RT-PCR
reverse transcription PCR
- W
white light
- WT
wild-type
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3106
References
- 1.Baskin TI, Briggs WR, Iino M. Can lateral redistribution of auxin account for phototropism of maize coleoptile? Plant Physiol. 1986;81:306–309. doi: 10.1104/pp.81.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iino M. Gravitropism and phototropism of maize coleoptiles: Evaluation of the Cholodny-Went theory through effects of auxin application and decapitation. Plant Cell Physiol. 1995;36:361–367. [Google Scholar]
- 3.Haga K, Iino M. Auxin-growth relationships in maize coleoptiles and pea internodes and control by auxin of the tissue sensitivity to auxin. Plant Physiol. 1998;117:1473–1486. doi: 10.1104/pp.117.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philippar K, Fuchs I, Lüthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Böttger M, Becker D, Hedrich R. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleland RE. The final action of hormones. In: Davies PJ, editor. Plant Hormones: Biosynthesis, Signal Transduction, Action! Dordrecht: Kluwer Academic Publishers; 2004. pp. 204–220. [Google Scholar]
- 6.Hager A, Menzel H, Kraus A. Experimente und hypothese zur primärwirkung des auxins beim streckungswachstum. Planta. 1971;100:47–75. doi: 10.1007/BF00386886. (Ger). [DOI] [PubMed] [Google Scholar]
- 7.Rayle DL, Cleland RE. The in-vitro acid-growth response: Relation to in-vitro growth responses and auxin action. Planta. 1972;104:282–296. doi: 10.1007/BF00386312. [DOI] [PubMed] [Google Scholar]
- 8.Rayle DL, Cleland RE. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hager A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J Plant Res. 2003;116:483–505. doi: 10.1007/s10265-003-0110-x. [DOI] [PubMed] [Google Scholar]
- 10.Bauer CS, Hoth S, Haga K, Philippar K, Aoki N, Hedrich E. Differential expression and regulation of K+ channels in the maize coleoptile: Molecular and biophysical analysis of cells isolated from cortex and vasculature. Plant J. 2000;24:139–145. doi: 10.1046/j.1365-313x.2000.00844.x. [DOI] [PubMed] [Google Scholar]
- 11.Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inzé D. superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King JJ, Stimart DP, Fisher RH, Bleecker AB. A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell. 1995;7:2023–2037. doi: 10.1105/tpc.7.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomine S, Lelièvre F, Boufflet M, Guern J, Barbier-Brygoo H. Anion-channel blockers interfere with auxin responses in dark-grown Arabidopsis hypocotyls. Plant Physiol. 1997;115:533–542. doi: 10.1104/pp.115.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marten I, Lohse G, Hedrich R. Plant growth hormones control voltage-dependent activity of anion channels plasma membrane of guard cells. Nature. 1991;353:758–762. [Google Scholar]
- 15.Zimmermann S, Thomine S, Guern J, Barbier-Brygoo H. An anion current at the plasma membrane of tobacco protoplasts shows ATP-dependent voltage regulation and is modulated by auxin. Plant J. 1994;6:707–716. [Google Scholar]
- 16.Keller CP, Van Volkenburgh E. The electrical response of Avena coleoptile cortex to auxins. Evidence in vivo for activation of a Cl− conductance. Planta. 1996;198:404–412. [Google Scholar]
- 17.Jones AM, Cochran DS, Lamerson MA, Evans ML, Cohen JD. Red light-regulated growth. Plant Physiol. 1991;97:352–358. doi: 10.1104/pp.97.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behringer FJ, Davies PJ. Indole-3-acetic acid levels after phytochrome-mediated changes in stem elongation rate of dark- and light-grown Pisum seedlings. Planta. 1992;188:85–92. doi: 10.1007/BF00198943. [DOI] [PubMed] [Google Scholar]
- 19.Jensen PJ, Hangarter RP, Estelle M. Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol. 1998;116:455–462. doi: 10.1104/pp.116.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian Q, Reed JW. Control of auxin-regulated root development by the Arabidiopsis thaliana SHY2/IAA3 gene. Development. 2001;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs I, Philippar K, Ljung K, Sandberg G, Hedrich R. Blue light regulates an auxin-induced K+-channel gene in the maize coleoptile. Proc Natl Acad Sci USA. 2003;100:11795–11800. doi: 10.1073/pnas.2032704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanderhoef LN, Briggs WR. Red light-inhibited mesocotyl elongation in maize seedlings. Plant Physiol. 1978;61:534–537. doi: 10.1104/pp.61.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iino M. Inhibitory action of red light on the growth of the maize mesocotyl: Evaluation of the auxin hypothesis. Planta. 1982;156:388–395. doi: 10.1007/BF00393308. [DOI] [PubMed] [Google Scholar]
- 24.Barker-Bridgers M, Ribnicky DM, Cohen JD, Jones AM. Red-light-regulated growth. Changes in the abundance of indoleacetic acid in the maize (Zea mays L.) mesocotyl. Planta. 1998;204:207–211. [Google Scholar]
- 25.Jones AM. Auxin-binding proteins. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:393–420. [Google Scholar]
- 26.Napier RM, David KM, Perrot-Rechenmann C. A short history of auxin-binding proteins. Plant Mol Biol. 2002;49:339–348. [PubMed] [Google Scholar]
- 27.Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 28.Dharmasiri N, Dharmasiris S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 29.Chen JG, Ullah H, Young JC, Sussman MR, Jones AM. ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev. 2001;15:902–911. doi: 10.1101/gad.866201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauly JM, Sealy IM, Macdonald H, Brearley J, Dröge S, Hillmer S, Robinson DG, Venis MA, Blatt MR, Lazarus CM, Napier RM. Overexpression of auxin-binding protein enhances the sensitivity of guard cells to auxin. Plant Physiol. 2000;124:1229–1238. doi: 10.1104/pp.124.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamagami M, Haga K, Napier RM, Iino M. Two distinct signaling pathways participate in auxin-induced swelling of pea epidermal protoplasts. Plant Physiol. 2004;134:735–747. doi: 10.1104/pp.103.031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones AM, Inn KH, Savka MA, Wu MJ, DeWitt NG, Shillito R, Binns AN. Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science. 1998;282:1114–1117. doi: 10.1126/science.282.5391.1114. [DOI] [PubMed] [Google Scholar]
- 33.Schwob E, Choi SY, Simmons C, Migliaccio F, Ilag L, Hesse T, Palme K, Söll D. Molecular analysis of three maize 22 kDa auxin-binding protein genes - transient promoter expression and regulatory regions. Plant J. 1993;4:423–432. doi: 10.1046/j.1365-313x.1993.04030423.x. [DOI] [PubMed] [Google Scholar]
- 34.Im KH, Chen JG, Meeley RB, Jones AM. Auxin-binding protein mutants in maize. Maydica. 2000;45:319–325. [Google Scholar]
- 35.Smith H. Light quality photoperception and plant strategy. Annu Rev Plant Physiol Plant Mol Biol. 1982;33:481–518. [Google Scholar]
- 36.Smith H. Physiological and ecological function within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:289–315. [Google Scholar]
- 37.Botto JF, Smith H. Differential genetic variation in adaptive strategies to a common environmental signal in Arabidopsis accessions: Phytochrome-mediated shade avoidance. Plant Cell Environ. 2002;25:53–56. [Google Scholar]
- 38.Carabelli M, Morelli G, Whitelam G, Ruberti I. Twilight-zone and canopy shade induction of the Athb-2 homeobox gene in green plants. Proc Natl Acad Sci USA. 1996;93:3530–3535. doi: 10.1073/pnas.93.8.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I. Shade avoidance response are mediated by the ATHB-2 HD-Zip protein, a negative regulator of gene expression. Development. 1999;126:4235–4245. doi: 10.1242/dev.126.19.4235. [DOI] [PubMed] [Google Scholar]
- 40.Morelli G, Ruberti I. Shade avoidance responses. Driving auxin along lateral routes. Plant Physiol. 2000;122:621–626. doi: 10.1104/pp.122.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murashige T, Skoog A. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 42.Fellner M, Horton LA, Cocke AE, Stephens NR, Ford ED, Van Volkenburgh E. Light interacts with auxin during leaf elongation and leaf angle development in young corn seedlings. Planta. 2003;216:366–376. doi: 10.1007/s00425-002-0881-7. [DOI] [PubMed] [Google Scholar]
- 43.Chen KH, Miller AN, Patterson GW, Cohen JD. A rapid and simple procedure for purification of indole-3-acetic acid prior to GC-SIM-MS analysis. Plant Physiol. 1988;86:822–825. doi: 10.1104/pp.86.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen JD, Baldi BG, Slovin JP. 13C6-[benzene ring]-indole-3-acetic acid: A new internal standard for quantitative mass spectral analysis of indole-3-acetic acid in plants. Plant Physiol. 1986;80:14–19. doi: 10.1104/pp.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller AN, Walsh CS, Cohen JD. Measurement of indole-3-acetic acid in peach fruits (Prunus persica L. Batsch cv Redhaven) during development. Plant Physiol. 1987;84:491–494. doi: 10.1104/pp.84.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wodzicki TJ, Abe H, Wodzicki AB, Pharis RP, Cohen JD. Investigation of the nature of the auxin-wave in the cambial region of pine stems. Validation of IAA as the auxin component by Avena coleoptile curvature assay and by gas chromatography-mass spectroscopy-selected ion monitoring. Plant Physiol. 1987;84:135–143. doi: 10.1104/pp.84.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen JD. Convenient apparatus for generation of small amounts of diazometane. J Chromatography. 1984;303:193–196. [Google Scholar]
- 48.Stahlberg R, Van Volkenburgh E. The effect of light on membrane potential, apoplastic pH and cell expansion in leaves of Pisum sativum L. var. Argenteum. Planta. 1999;208:188–195. [Google Scholar]
- 49.Katekar GF, Geisler AE. Auxin transport inhibitors IV. Evidence of a common mode of action for a proposed class of auxin transport inhibitors, the phytotropins. Plant Physiol. 1980;66:1190–1195. doi: 10.1104/pp.66.6.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- 51.Huisinga B. Influence of light on growth, geotropism and guthation of Avena seedlings grown in total darkness. Acta Bot Neerl. 1964;13:445–487. [Google Scholar]
- 52.Huisinga B. Influence of irradiation on the distribution of growth in dark-grown Avena seedlings. Acta Bot Neerl. 1967;16:197–201. [Google Scholar]
- 53.Huisinga B. The export of auxin from tips and from sections of Avena coleoptiles as influenced by R. Acta Bot Neerl. 1976;25:313–320. [Google Scholar]
- 54.Naqvi SM. Kinetics of auxin transport in light and in dark grown Zea mays L. coleoptile segments. Zeitschrift Pflanzenphysiol. 1975;76:379–383. [Google Scholar]
- 55.Tsiantis M, Brown MIN, Skibinski G, Langdale JA. Disruption of auxin transport is associated with aberrant leaf development in maize. Plant Physiol. 1999;121:1163–1168. doi: 10.1104/pp.121.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garbers C, DeLong A, Deruere J, Bernasconi P, Soll D. A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 1996;15:2115–2124. [PMC free article] [PubMed] [Google Scholar]
- 57.Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- 58.Foster RJ, McRae DH, Bonner J. Auxin-antiauxin interaction at high auxin concentrations. Plant Physiol. 1955;30:323–327. doi: 10.1104/pp.30.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evans ML, Hokanson R. Timing of the response of coleoptiles to the application and withdrawal of various auxins. Planta. 1969;85:85–95. doi: 10.1007/BF00387663. [DOI] [PubMed] [Google Scholar]
- 60.De Klerk GJ. Hormone requirements during the successive phases of rooting of Malus microcuttings. In: Terzi M, editor. Current issues in plant molecular and cellular biology. Dordrecht: Kluwer Academic Publishers; 1995. pp. 111–116. [Google Scholar]
- 61.Heupel T, Stange L. The auxin antagonist p-Chlorophenoxyisobutyric acid abolishes polar distribution of DNA synthesizing cells within the meristem of Riella helicophylla. J Plant Physiol. 1995;146:757–759. [Google Scholar]
- 62.Sugaya S, Ogmiya A, Kikuchi M, Hayashi T. Isolation and characterization of a 60 kDa 2,4-D-binding protein from the shoot apices of peach trees (Prunus pessica L.); It is a homologue of protein disulfide isomerase. Plant Cell Physiol. 2000;41:503–508. doi: 10.1093/pcp/41.4.503. [DOI] [PubMed] [Google Scholar]
- 63.Niedergang-Kamien E, Leopold AC. The inhibition of transport of indoleacetic acid by phenoxyacetic acid. Physiol Plant. 1959;12:776–785. [Google Scholar]
- 64.Hertel R, Evans ML, Leopold AC, Sell HM. The specificity of the auxin transport system. Planta. 1969;85:238–249. doi: 10.1007/BF00389401. [DOI] [PubMed] [Google Scholar]
- 65.Tsai DS, Arteca RM. Inhibition of IAA-induced ethylene production in etiolated mung bean hypocotyl segments by 2,3,5-triiodobenzoic acid and 2-(p-chlorophenoxy)-2-methyl propionic acid. Physiol Plant. 1984;62:448–452. [Google Scholar]
- 66.Davies PJ. The plant hormone concept: Concentration, sensitivity and transport. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht: Kluwer Academic Publishers; 1995. pp. 13–38. [Google Scholar]
- 67.Felle H, Peters W, Palme K. The electrical response of maize to auxin. Biochem Biophys Acta. 1991;1064:199–204. doi: 10.1016/0005-2736(91)90302-o. [DOI] [PubMed] [Google Scholar]
- 68.Ray PM, Dohrman U, Hertel R. Characterization of naphthaleneacetic acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977;60:585–591. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimomura S, Inohara N, Fukui T, Fukui M. Different properties of two types of auxin-binding sites in membranes from maize coleoptiles. Planta. 1988;175:558–566. doi: 10.1007/BF00393079. [DOI] [PubMed] [Google Scholar]
- 70.Jones AM, Lamerson P, Venis MA. Comparison of site I auxin binding and a 22-kilodalton auxin-binding protein in maize. Planta. 1989;179:409–413. doi: 10.1007/BF00391088. [DOI] [PubMed] [Google Scholar]