Abstract
Phosphate limited grown Anabaena variabilis has the capability of processing information about external phosphate fluctuations by means of interconnected adaptive events. Adaptive events are physiological processes that are characterized by two opposite manifestations, namely adapted states and adaptive operation modes. In adapted states the energy-converting constituents of the uptake system operate under the prevailing external conditions in a coherent manner with least energy dissipation. Adaptive operation modes take place when adapted states are disturbed by persistent changes in phosphate supply. In this mode the outcome of former adaptations to elevated phosphate levels guides the emergence of a new adapted state. The influence of antecedent adapted states on subsequent adaptations was studied experimentally and characteristic examples for such information processing are given. The theory of self-referential systems allowed analyzing these examples. For this purpose adaptive events had to be considered as elements of a communicating network, in which, along a historic succession of alternating adapted states and adaptive operation modes, information pertaining to the self-preservation of the organism is transferred from one adaptive event to the next: the latter “interprets” environmental changes by means of distinct adaptive operation modes, aimed at preservation of the organism. The result of this interpretation is again leading to a coherent state that is passed on to subsequent adaptive events. A generalization of this idea to the adaptive interplay of other energy converting subsystems of the cell leads to the dynamic view of cellular information processing in which the organism recreates itself in every new experience.
Key Words: adaptation, cyanobacteria, information processing, phosphate uptake, self-referential systems
Introduction
Under phosphate limiting growth conditions cyanobacteria develop the capability of interpreting fluctuations in the external phosphate concentration as a signal for instigation of a distinct adaptive behavior, in which the phosphate uptake system is reconstructed1–3 and information about preceding phosphate exposures is processed.4,5 The biological significance of this behavior can be deduced from the nutrient situation in oligotrophic lakes, in which the formation of phytoplankton biomass is limited by the amount of inflowing phosphate.6 Under this condition the phosphate concentration decreases to such low levels that the uptake system has not sufficient energy to overcome the existing concentration gradient across the cell membrane.2,3 In such a situation cyanobacteria and algae can only grow, when the external concentration exceeds, at least occasionally, a characteristic threshold value above which available energy suffices for the transport into the cell. Phosphate is then incorporated at a high rate and stored in the form of polyphosphate granules that serve as the actual phosphorus source for the proliferating cell. Usually the growth rate is the higher, the more polyphosphate had been accumulated in the past.7
This uptake and storage mechanism has an important consequence for the response of the algal cells to alterations of phosphate supply. Due to the uptake activity of the whole community, the concentration of supplied phosphate in the external milieu decreases rapidly to the prevailing threshold value again, which, under phosphate-deficient conditions, is in the nanomolar range.8,9 As a result of this energetic constraint, occasional occurring rises in the external concentration are interrupted by periods without phosphate supply, so that fluctuations of the ambient phosphate concentration are “experienced” by the cells as a pattern of pulses. In this scenario the storage mechanism described above confronts the cells with a regulatory problem: on the one hand, sufficient phosphate for sustaining continuous growth has to be incorporated during each pulse. On the other hand, the size of the polyphosphate granules must not become so big that cellular structures are disrupted. This problem can only be solved when the uptake system “knows” during each pulse how much phosphate has been incorporated during foregoing pulses. A direct influence of the size of the granules on the phosphate carrier is hard to imagine since the polyphosphate granules are segregated in the cytoplasm and osmotically inactive. But the activity of the carrier (as well as the growth rate) can be indirectly conformed to the polyphosphate content, if some sort of cellular information processing guides the adaptive behavior of the phosphate uptake system during each pulse, such that the amount of accumulated phosphate and the growth rate are constantly conformed to each other.
We have previously shown that the phosphate uptake system of the filamentous cyanobacterium Anabaena variabilis is capable of processing information about alterations in phosphate supply.4,5 The present article is a continuation of this study. Investigating under which conditions an alteration in the external concentration is converted into a signal for a distinct adaptive behavior, we demonstrate that cyanobacteria are able of discriminating between different patterns of phosphate pulses by diverse adaptive responses. In each of these responses the uptake system attains a distinct adapted state that differently affects subsequent adaptations to changing phosphate concentrations. The connectivity of adapted events is discussed in respect to information processing about environmental changes.
Materials and Methods
Growth conditions.
Phosphate limited growth of the cyanobacterium Anabaena variabilis Kützing (Algal Culture Collection Göttingen, strain 1403-4b) was performed in a discontinuous cultivation mode in 300 mL cultures. The medium contained 1 mM KCl, 0.3 mM MgSO4, 0.25 mM CaCl2, 0.1 mM EDTA and was supplemented with 5 mM NaHCO3, 2.5 µM Fe-EDTA and the trace element solution of medium D of Kratz and Myers.10 The culture was illuminated with fluorescent lamps at a light intensity of about 250 µE·m−2·s−1 and bubbled with air and 5% CO2. Discontinuous growth was achieved as follows: Every 24 hours an aliquot of the culture was discarded and replaced by fresh medium again containing a limiting amount of K2HPO4 (usually 10 µM, if not indicated otherwise). The remaining cyanobacteria incorporated freshly added phosphate within 30 min and the external concentration decreased to a threshold value, which, under these conditions was in the nanomolar range. The cells then grew at the expense of stored phosphate. For this reason the total phosphorus content in the culture and the proliferation of the cyanobacterial population could be adjusted by the amount of added fresh medium: for example, when 150 mL of the original 300 mL culture had been replaced by new medium, the cells doubled once during subsequent growth, when 225 mL were replaced, the cells doubled approximately twice.
Characterization of the adaptive behavior of the uptake system.
In general, we studied the uptake process by means of 32P-phosphate, because adaptive phenomena usually occur at concentrations below the limits of detection by conventional analytical methods. The incubation medium contained the same constituents as the growth medium with the addition of 5 mM Hepes/KOH (pH 7.9), but lacked the trace elements and Fe-EDTA. During the uptake process the cyanobacteria were exposed in thermostated and magnetically stirred incubation vessels to the same light intensity and temperature as during growth. 32P-phosphate was supplied by NEN (370 MBq·mL−1) and used after appropriate dilution with non-radioactive phosphate. The adaptive properties of the phosphate uptake system of phosphate deficient populations were analyzed by two different methods in which an alteration of the influx of tracer was related to concomitantly occurring adaptive changes of the uptake system. In the first method the influx of tracer was followed under conditions under which phosphate deficient cells were exposed to a sudden increase in the external concentration that was then maintained at a constant level. In this case the time course of tracer influx revealed how rapidly the uptake system altered its properties during exposure to the external concentrations employed. For this method the algal suspensions had to be sufficiently diluted so that the uptake process itself practically did not alter the external concentration. In the second method, using less diluted suspensions of the original culture, the time course of the decrease of the external phosphate concentration was analyzed. This method was employed for investigating the adaptive behavior in a sequence of repeated additions of phosphate. In both methods the time course of phosphate incorporation was followed by filtering aliquots of the sample in regular time intervals and measuring the radioactivity on the filter (first method) or in the filtrate (second method).
In the experiments presented in this paper the quantity of incorporated tracer usually exceeded that of the exchangeable phosphate pool. For this reason a possible isotopic exchange taking place concomitantly with the net uptake process was negligible.
Analysis of uptake kinetics.
In 1970 Michel Thellier suggested analyzing the concentration dependence of mineral salt adsorption by plants by plotting uptake rates vs. the logarithm of the external mineral salt concentration. In such a plot the resulting curve intercepts the x-axis at the logarithm of a threshold concentration at which uptake ceases for energetic reasons. As has been shown by Thellier in several papers,11–13 the resulting curves revealed essential features of the dependence of uptake rates on the driving force of this process: at concentrations close to the equilibrium (threshold) concentration the data obeyed a linear relation between uptake rates and the logarithm of the external concentration. This could be explained by a proportional dependence of the influx flow on the driving force close to equilibrium, in accordance with general principles of non-equilibrium thermodynamics. In contrast, a nonlinear dependence of uptake rates on the logarithm of the external concentration appeared at salt concentrations that exceeded significantly the threshold value, in regions far from the equilibrium. Occasionally however, the proportional relationship between flows and forces extended to higher concentrations.
In an application of this approach for analysing the adaptive properties of the phosphate uptake system of the unicellular cyanobacterium Anacystis nidulans, a distinction between the linear and the nonlinear dependence of the uptake rate on the logarithm of the external concentration turned out to be of fundamental importance.1,2,14 When the uptake system was adapted to concentrations that were much higher than the threshold value, a linear relationship extended from the threshold value to high external concentrations. In contrast, under nonadapted conditions under which the uptake system even adapted to the external concentrations employed in the experiment during the course of the investigation, a non-linear dependence was observed. A similar behavior was noticed with Anabaena variabilis (see below). For this reason the time course of the decrease in the external concentration was analyzed in stable adapted states using the simple linear function:
d[Pe]/dt = − LP (ln[Pe] − ln[Pe]A).
In cyanobacteria 2.3ln[Pe]A= −nP ΔpH − logK,2,3 where [Pe] is the external concentration, [Pe]A represents the threshold value and K the equilibrium constant for polyphosphate formation. LP is a conductivity coefficient that reflects the activity of the phosphate carrier.1,2 Conversely, the time course of the decrease in the external concentration in the transient adaptive operation mode was analyzed by the full expression, proposed by Thellier, consisting of a linear and a nonlinear term:
Jp = d[Pe]/dt = −LP(ln[Pe] − ln[Pe]A) − L(ln[Pe] − ln[Pe]A)m with m > 1 (see legends).
Preferably, fitting has been performed in two steps. First the linear term was fitted with data close to equilibrium. After insertion of the parameters of the linear function into the whole expression, the parameters of the non-linear term were determined.
Every experiment is a representative example of at least five independent experiments. Curve fitting was performed using the MLAB computer program. The reliability of the method was checked using two independent samples and following the time course of phosphate removal from the external medium in either sample. Curve fitting of a plot of the data from both time courses yielded the same value of R2 (0.993, n = 12) as the individual time courses of the two independent samples (R2 = 0.994 and 0.996 resp.). The fitted parameters are given in the legends; the obtained R2 were usually above 0.99.
Results
The time course of 32P-phosphate influx as a mirror of adaptive properties of the phosphate uptake system.
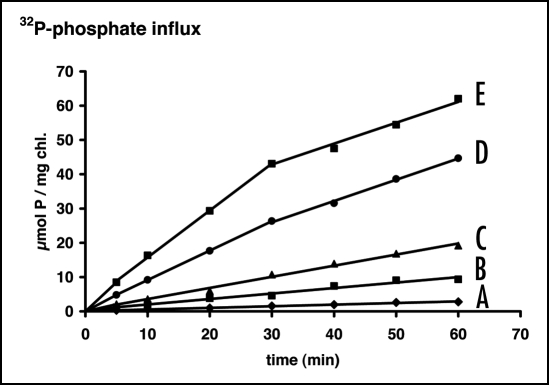
The adaptive response of the uptake system of a phosphate deficient population of A. variabilis was investigated, raising the external concentration abruptly and maintaining it at a higher level. As shown in Figure 1, the time course of 32P-phosphate influx into the polyphosphate pool was linear at lower concentrations, indicating that in this case the uptake system did not alter its properties during the measurement conditions. At higher concentrations, however, the time course of 32P-phosphate influx revealed a biphasic shape. Here a higher initial velocity gradually decreased during a transition period of about 30 minutes to a lower value that then remained constant for about half an hour. Apparently during this transition period an adaptive reconstruction of the uptake system took place, leading to a stable state that was preserved for a certain period of time.
Figure 1.
Time course of 32P-phosphate influx in A. variabilis (cultivated on a total phosphorus content of 5 µmol·L−1) at different external concentrations. (A) 20 nM; (B) 100 nM; (C) 200 nM; (D) 500 nM; (E) 1 µM. For the measurement the original suspension was diluted 500-fold to ensure that the uptake process did not alter the external concentration.
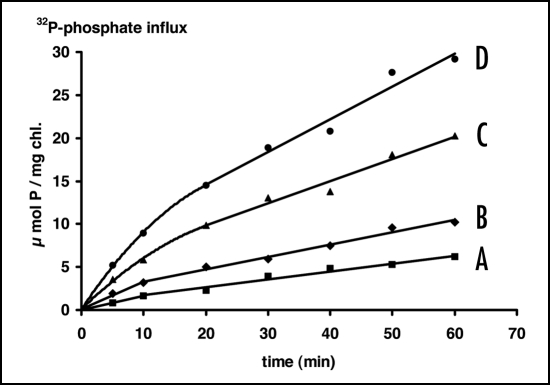
The critical concentration, above which an adaptive reconstruction of the uptake system could be observed, depended on the height of the phosphate concentration to which the population had been exposed during the foregoing growth. When A. variabilis was supplied during discontinuous growth with a lower amount of phosphate (0.1 µM instead of 5 µM, as in the experiment above), a reconstruction of the uptake system already occurred at lower concentrations (Fig. 2). We may conclude that from the adaptive behavior at different external concentrations information can be obtained about the height of the phosphate pulses to which a population had been exposed during preceding growth. The difference between Figure 1 and Figure 2 also shows that the adaptive reconstruction was faster in cells originating from lower growth concentrations.
Figure 2.
Time course of 32P-phosphate influx in A. variabilis, (cultivated on a total phosphorus content of 0.1 µmol·L−1) at different external concentrations. (A) 20 nM; (B) 50 nM; (C) 200 nM; (D) 500 nM. Experimental conditions as in Figure 1.
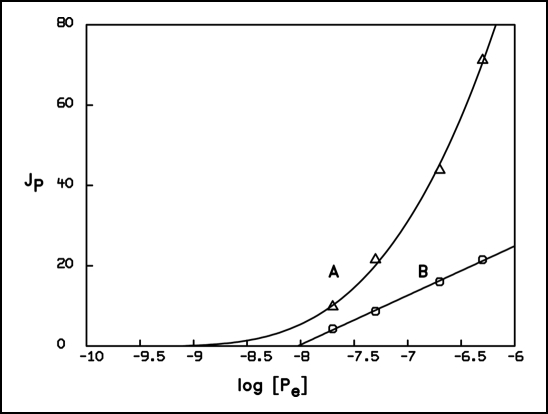
Thellier plots of uptake rates JP vs the logarithm of the external phosphate concentration, obtained from Figures 1 and 2, revealed a characteristic difference between the initial velocities and the subsequent steady state uptake rates. When, for example, the initial velocities from Figure 2 that specify the uptake system before attainment of adapted states, were plotted against the respective logarithmic concentrations, the resulting function showed an upwards-curved shape (Fig. 3). In contrast, steady state rates, established after adaptation to elevated concentrations, obeyed a linear dependence of Jp on log[Pe] over a wide concentration range. A similar behavior has been previously observed with the unicellular cyanobacterium Anacystis nidulans.1,2,14 We therefore may conclude that only adapted states of the uptake system of these organisms are characterized by an extended range of validity of the linear relationship between the flux of phosphate into the cell and the driving force for this process. The curve fitting for the time course of the decrease of the external concentration has therefore been interpreted accordingly (see below).
Figure 3.
Thellier plots of initial uptake velocities (curve A) and of steady state uptake rates in adapted states (curve B) vs the logarithm of the respective concentrations; Graph A obtained from the initial velocities of Figure 2 was fitted using the non linear equation: JP= 1.0 · (log[Pe] − log(0.74 · 10−9)) + (3.0 · (log[Pe] − log(0.74 · 10−9))3, whereas graph B obtained from the steady state rates of Figure 2 was fitted using the linear function JP = 12.3 · (log[Pe] − log(9.38 · 10−9)). The phosphate uptake rate JP is given as µmol · (mg chl.·h)−1, the logarithm of the external phosphate concentration is expressed relative to the unit standard concentration.
In the experiments of Figures 1 and 2 the suspension employed was sufficiently diluted, so that the amount of incorporated phosphate did not significantly alter the external concentration. If, however, a less diluted suspension was used, the external concentration did not remain constant, but decreased more or less rapidly (dependent on the number of cells in the incubation medium), until the threshold value was attained (here we were dealing with an experimental setup that more reflects the natural situation under which a community is exposed to pulsewise occurring rises in the ambient concentration). In this case the adaptive alterations of the uptake system can be expected to depend in a complex manner on the number of cells in the incubation medium and the height of the external concentration at the beginning of the uptake process, for the following reason: the lower the cell number, the slower the decrease of the external concentration, the more time for the population to adapt; the higher the initial concentration, the greater the adaptive response.
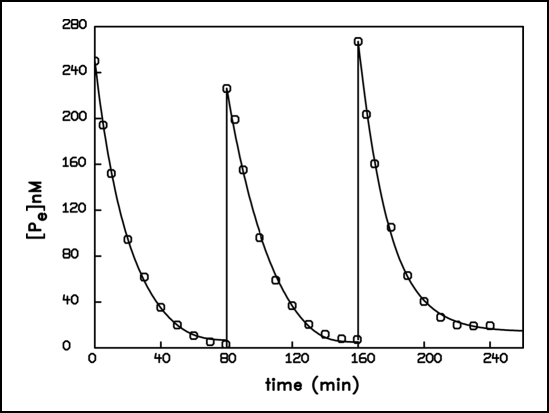
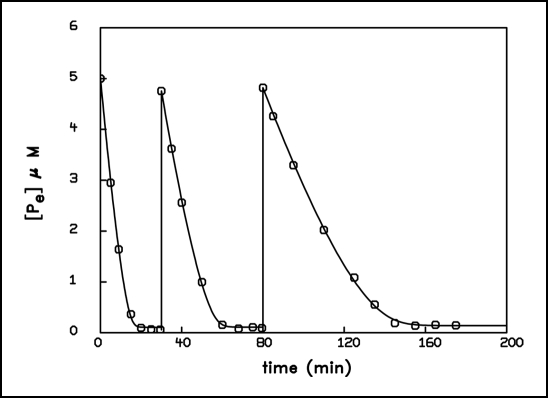
To study these complex features of the uptake behavior, we exposed two different dilutions of the same population of A. variabilis to several pulses, such that the experimentally supplied amount of phosphate in each pulse and the original phosphorus content of the suspension was of the same magnitude. Figures 4 and 5 show the kinetics of phosphate removal by two differently diluted algal suspensions (corresponding to total bacterial phosphorus content of 0.25 and 5 µmol·L−1 resp. The culture for this experiment was again grown on total phosphorus content of 10 µmol·L−1). In accordance with Figure 1 no adaptation was to be expected and none was observed at an external concentration of 0.25 µM, since here the initial concentration was below the level that provokes an adaptive transition. The shape of the first two pulses was practically identical and a fit using the nonlinear flow force relationship yielded similar parameters. Only the third pulse displayed deviations. We may conclude that under this condition the cells never attained a persisting new adapted state during the uptake process. In contrast, when the 20 times thicker suspension was exposed three times to an external concentration of 5 µM (which was in the range in which adaptive transitions were observed), the adaptive kinetics varied much more from pulse to pulse. Here the population developed, during the first pulse, a distinct adapted state that could be fitted by a linear flow force relationship. This state appeared to be preserved after the concentration had decreased to the threshold value again, so that the subsequent uptake behavior in the second pulse showed new properties in a fit by the semilogarithmic relationship. The same happened in the third pulse. Thus, although the total phosphorus content in each of these two populations had tripled after the third pulse, the kinetics of the more diluted sample remained practically unchanged, whereas the kinetics of the less diluted suspension were considerably modified.
Figure 4.
Time course of 32P-phosphate removal from the external medium during three consecutive pulses of 250 nM phosphate. The content of bacterial phosphorus of the suspension of A. variabilis was likewise 250 nmol·L−1. The curves represent the best computer fit using the non linear equation: d[Pe]/dt = − 1 · (ln[Pe]−ln[Pe]A) − L · ( ln[Pe]−ln[Pe]A)m, with the parameters: L = 0.013 nM min−1, [Pe]A = 6.42 nM, m = 5 (first pulse); L = 5.64 ·10−3 nM min−1, [Pe]A = 4.95 nM, m = 5 (second pulse); L = 0.397 nM min−1, [Pe]A = 14.26 nM, m = 3 (third pulse).
Figure 5.
Time course of 32P-phosphate removal from the external medium during three consecutive pulses of 5 µM. The content of bacterial phosphorus of the suspension of A. variabilis was likewise 5 µmol·L−1. Experimental conditions as in Figure 4. The curves represent the best computer fit using the equation: d[Pe]/dt = −Lp · (ln[Pe] − ln[Pe]A) with the parameters: Lp = 0.111 µM min−1, [Pe]A = 0.108 µM, (first pulse); Lp = 0.061 µM min−1, [Pe]A = 0.110 µM, (second pulse); Lp = 0.029 µM min−1, [Pe]A = 0.14 µM, (third pulse).
The adaptive behavior as an index of the properties of foregoing phosphate pulses.
The experimental setup described above allowed to answer two questions: first, are cells capable of discriminating between two different pulse patterns in a way that differently affects a subsequent adaptive response, even if they are exposed to the same pulses, but in nonidentical order? Second, do distinct adapted states differently affect subsequent adaptations after cell division?
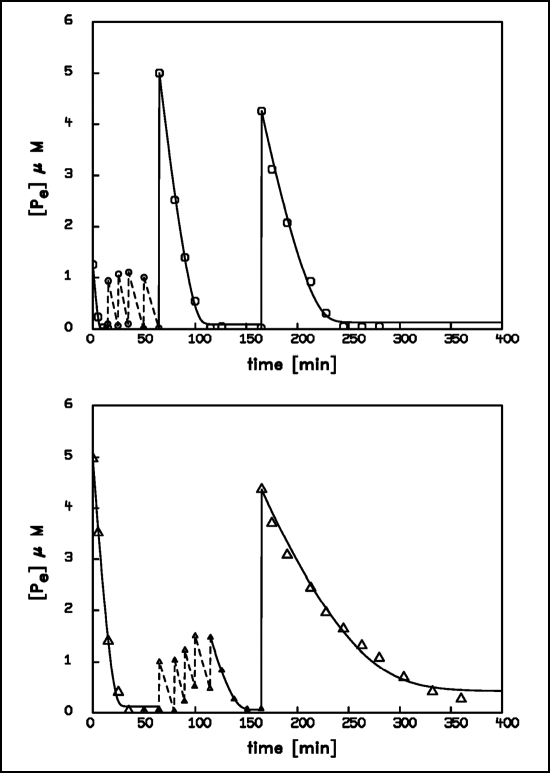
In order to answer the first question, we have exposed two samples of the same population of A. variabilis to five small pulses of 1 µM each and one greater pulse with 5 µM, but in reverse order: one sample experienced first the five smaller pulses and then the great pulse, the other sample vice versa. A comparison of the uptake kinetics in a subsequent pulse of identical maximum concentration revealed significant differences (Fig. 6), indicating a more pronounced response of the sample that experienced the smaller pulses after the great pulse. We have also performed this experiment with a lower number of small pulses and obtained essentially the same result, however, the difference in the resulting adaptive behavior was the smaller, the lower the number of pulses (data not shown). This indicates that under this condition a difference in the pattern of phosphate fluctuations leads to a corresponding difference in the subsequent adaptive behavior. Hence, we may conclude that already a simple prokaryotic organism is capable of some sort of pattern recognition and to transcribe information about former environmental alterations into the form of the subsequent adaptive responses.
Figure 6.
Time course of 32P-phosphate removal from the external medium by two identical populations of A. variabilis; (upper graph): 5 pulses of 1 µM followed by 1 pulse of 5 µM; (lower graph): 1 pulse of 5 µM followed by 5 pulses of 1 µM. For the small pulses only the concentration before and after addition of phosphate are given in the graph. The two different patterns initiate a different adaptive behavior during the final pulse. The curves of the final pulse represent the best computer fit using the equation: d[Pe]/dt = −Lp · (ln[Pe] − ln[Pe]A). The following parameters were obtained: upper graph: Lp = 0.026 µM min−1; [Pe]A = 0.13 µM; lower graph: Lp = 0.018 µM min−1; [Pe]A = 0.42 µM.
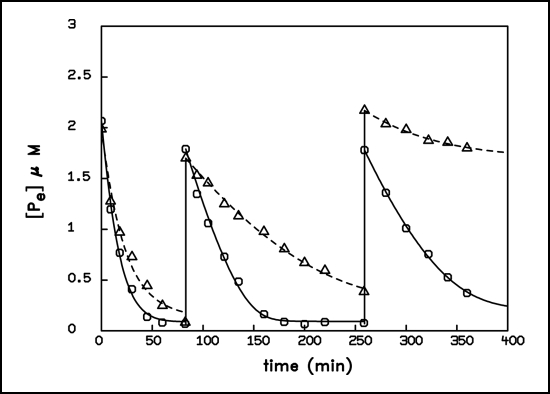
When the two patterns of phosphate pulses, to which two different samples of the same population were exposed, do differ significantly, a distinct adaptive behavior could even be observed after subsequent cell division, indicating that here information about former nutrient fluctuations is transferred to daughter generations. In the following we present an example in which we have challenged two identical suspensions of A. variabilis with two different supply modes: one suspension experienced one pulse of 10 µM, the other ten pulses of 1 µM. After this treatment the two suspensions were cultivated for 24 hours at the expense of the stored phosphorus, which was the same in both cultures. Thereby, the chlorophyll content of the two cultures increased from 0.13 to 0.33 and 0.31 mgL−1resp., indicating that the number of cells had tripled during that time. The two suspensions were then harvested and exposed to three identical test pulses for a comparison of their adaptive behavior. Figure 7 shows that the culture that had been challenged by one great pulse (high pulse culture) during its previous growth deactivated the uptake system more rapidly than the reference culture (low pulse culture) in which the external concentration never attained high values. Table 1 gives a summary of the fitted flow-force parameters. In a sequence of pulses the two cultures differ significantly in respect to alterations of threshold values and conductivity coefficients of the linear term. Whereas the ‘low pulse culture’ only showed a slight increase of the threshold value, the rise of this parameter was dramatic in the ‘high pulse culture’. Even more interesting was the fate of the conductivity coefficient: it practically remained constant in the ‘low pulse culture’, but decreased in the ‘high pulse culture’ in the second pulse by almost one order of magnitude. In the third pulse, however, it increased in this culture again, concomitantly with a heightening of the threshold value, indicating that here a major reconstruction of the uptake system took place. It appears as if the cells of the ‘high pulse culture’ “remember” the former challenge by elevated phosphate concentrations, several cell divisions ago and; as a result they seem to anticipate during subsequent adaptations to pulses with a higher maximum concentration a continuation of excessive phosphate supply.
Figure 7.
Time course of 32P-phosphate removal from the external medium by two populations of A. variabilis, produced by different pretreatments of the same mother culture. Circles: low pulse culture, triangles: high pulse culture, see text. The curves represent the best computer fit using the equations: First pulse: d[Pe]/dt = −0.01 · (ln[Pe] − ln[Pe]A) − L · (ln[Pe] − ln[Pe]A)3 for the low pulse culture and d[Pe]/dt = −0.004 · (ln[Pe] − ln[Pe]A) − L · (ln[Pe] − ln[Pe]A)3 for the high pulse culture. All other pulses: d[Pe]/dt = −Lp · (ln[Pe] − ln[Pe]A). The parameters are given in Table 1.
Table 1.
Synopsis of the parameters from Figure 7, characterizing the uptake behavior in three consecutive phosphate pulses of the two differently pretreated cultures
| Culture | |||
| Parameter | Pulse Number | Low Pulse | High Pulse |
| LP | First | 0.0100 | 0.0400 |
| Second | 0.0112 | 0.0062 | |
| (µM·min−1) | Third | 0.0096 | 0.0256 |
| L | First | 0.0025 | 0.0026 |
| Second | 0 | 0 | |
| (µM·min−1) | Third | 0 | 0 |
| [Pe]A (µM) | First | 0.089 | 0.105 |
| Second | 0.093 | 0.261 | |
| Third | 0.205 | 1.690 | |
When the high and low pulse cultures were further cultivated for prolonged time on low phosphate concentrations, the difference in the adaptive behavior was lost and the uptake system passed again into an adaptive mode. Naturally the system also revealed a new adaptive behavior when an adapted state was disturbed by an increase of the phosphate concentration to levels to which the system was no longer conformed.
Discussion
Interpretation of adaptive events as possible elements of a cellular communication.
A sudden rise of the external concentration to micromolar levels initiates an adaptive reconstruction of the uptake system. The adaptive response to increases in the external concentration was studied analyzing the time course of 32P-phosphate influx at different but constant external concentrations. This established that adaptive processes depend on previous adaptations to external phosphate concentrations in the growth medium: cyanobacteria that had been exposed to a higher concentration for a certain period of time during their growth required later higher concentrations for an onset of the adaptive process. They also needed more time for an adaptive reconstruction than organisms originating from cultures with a lower growth concentration.
The dependence of an adaptive response on former adaptations reveals complex features when the adaptive process is studied under conditions under which the external concentration is not kept at a constant level, but also decreases as a result of the uptake activity of the whole population, as is the case in a natural habitat. In this situation a distinct pattern of pulses incites to distinct adaptive behavior, indicating that cyanobacteria are capable of discriminating between different environmental phosphate fluctuations; stored information about these differences is then used for the regulation of subsequent adaptive responses in an anticipatory manner (see Fig. 7). An investigation of this phenomenon, however, necessitates a research strategy that departs from traditional objectivistic approaches, for the following reason: in a study of the adaptive behavior the experimentalist is part of the investigated system and of the organismic response to the selected experimental conditions. In the present case the experimentalist determines, by an appropriate choice of the cell number in the incubation medium, how fast after phosphate supply the external concentration decreases to the threshold value again. This decides on the endurance of the cells to elevated phosphate levels and, in consequence, whether or not an alteration in the external phosphate concentration is “interpreted” by cells as a signal for the onset of an adaptive response. The self-referential dependence of the investigated phenomenon on the way the investigation has been performed is a general feature of complex metabolic networks in that “effects occur relative only to the context in which they are experienced and experimental conditions are an essential part of it”.15
Setting out from the observed response of cyanobacteria to phosphate fluctuations, it is now possible to discuss how the experience of an environmental alteration contributes to the self-constitution of the cell. For this purpose it is useful to employ the notion of information, originally given by Bateson.16 This notion accounts for a relation between the self-organisation of a system and information processing in the respective environment. Accordingly, an elementary unit of information is definable as a difference (in the environment) that makes a difference (in the self-constitution of the system). The relevance of this definition for the analysis of the adaptive behavior becomes evident when adaptive events are considered as elements of a communicating system. We have shown that adaptive events are characterized by two opposite manifestations, namely adapted states and adaptive operation modes.5,17 The former emerge when an adaptive process results in an intermediary stable state; they can be described by objective parameters, obtained from studies under defined experimental conditions. The latter arise, when an adapted state is disturbed by an environmental alteration. An adaptive operation mode comprises all translational and post-translational modifications of the cell, by which the properties of the energy dependent uptake system are recoordinated with the overall energy conversion of the cell. This process gives rise to a new type of cell, which again functions in a coherent manner, but displays a different composition.3 Hence, in an adaptive operation mode the system “interprets”, in dependence on former experiences, a perceived difference in the environment by developing a difference in the cellular structure. During an experimental analysis of this mode the organisms also adapt to the experimental conditions during the course of an investigation, making futile any analysis in objectivistic terms (see above). On the condition that an adapted state is regarded as an objective manifestation of a subsystem and the adaptive operation mode as its “subjective” behavior, adaptive events can be treated as elements of an ongoing cellular communication, based on alternations of those “subjective” and “objective” manifestations. Naturally this interpretation is only justified when information about the system in its prevailing environment is actually processed by transitions between adapted states and adaptive modes. The experiments presented above allow an interpretation along this line. They show that adaptive events have a temporal vector character in that an adaptive reconstruction, aimed at optimal performance in the future,18 is guided by information about previously experienced patterns of phosphate pulses. The fact that an experimental observation is shaped by the experimental protocol is consistent with this idea, since the prevailing experimental conditions also constitute information that is processed. In the following section we will elaborate in more detail, why ‘objective and subjective’ manifestations of the uptake system are a necessary consequence of the kinetic and energetic properties of its constituents.
The kinetic and energetic properties of adapted states.
For a discussion of possible information processing by energy converting subsystems, it is necessary to consider the energetic constraints that determine the incorporation of external phosphate into the polyphosphate pool. The incorporation process comprises three steps: first, the transport of phosphate into the cell, second, its subsequent conversion to ATP, and third, polyphosphate formation from ATP. Phosphate transport into the cell is potentially energy dependent and driven by an ATPase in the cell membrane.19 Also the next step, the conversion of transported phosphate to ATP, driven by the proton motive force at the thylakoid membrane, requires energy. Only the subsequent transfer of the terminal phosphate group from ATP to the polyphosphate chain takes place without energy consumption. In adapted states energy dissipation is minimal.8,9 In theory, energy converting systems can attain two stable states of minimal entropy production: one state exists at the threshold value where the driven flows vanish and hence no work is invested in the incorporation process; in Stucki's terminology, the system functions here “without load”.20 The other state arises, when the involved energy converters operate with optimal efficiency (here the system works “with load”20). In this state the degree of coupling between the phosphate flow into the cell and the ATPase reaction on the one hand and the H+/ATP stoichiometry of the ATP synthase on the other hand are tuned like a sensor to the prevailing external and cytoplasmic phosphate concentrations,19 such that incorporation of external phosphate into polyphosphates is performed with optimal efficiency9 (naturally the adjusted optimal efficiency is below the maximal possible efficiency, corresponding to the state of thermodynamic equilibrium8,20). These accommodations are not only important for the storage of information about former phosphate exposures (see below), but also regulate the height of the threshold value, which is the higher, the lower the degree of coupling between the transport system and the ATPase. Furthermore, in adapted states a linear relationship exists between the flows and forces that determine the operation of these energy converting subsystems.14 When these conditions are fulfilled, the concentration dependence of the uptake rate obeys, over a wide concentration range, the linear relationship between uptake rate and the logarithm of the external concentration, proposed by Thellier.11 The validity of the semilogarithmic function ranges from the threshold value to the external concentration, to which the system has been adapted.14 It is notable that the resulting linear function
JP=LP·(log([Pe]−log[Pe]A)
has the same structure as Weber-Fechner's law, when the uptake rate JP is interpreted as the sensory response to the stimulus [Pe]. Apparently in adapted states of cyanobacteria the relation between stimulus and response follows a similar logic structure as sensory perception in higher organisms. The function allowed for a prediction, within its range of validity, of the uptake rate for any external concentration and adapted state.9
Possible information storage by adapted states.
The “linear behavior” is maintained for a prolonged period of time, when the external concentration has decreased to the threshold value again.1,2 This is a precondition for the observed transfer of information from one pulse to the next. At the threshold value an intermediary stable state “without load” preserves the energetic and kinetic properties of the stable state, attained during operation “with load”.20 The stability of the system at the threshold value is further supported by a mutual adjustment of the transport system and the ATPase. Thereby, the transport system is conformed to a stable state of minimum entropy production at the existing gradient between the stationary cytoplasmic phosphate concentration and the threshold value, which, in turn, is maintained by these conformed properties. A similar adjustment seems to exist between the stationary cytoplasmic phosphate concentration, resulting from the energetic and kinetic properties of the F-ATPase at the thylakoid membrane. In energetic terms the KM of this enzyme is adapted to the steady state concentration, determined by the respective H+/ATP-stoichiometry.19 In kinetic terms the conformed rate of the ATP-synthase must sustain growth at a rate, which is anticipated in the light of previous exposures.5 In this way the properties of this self-referential ensemble reflect the preceding growth conditions, under which this ensemble had been established. As a result the characteristic attributes of the self-referential state of the uptake system are arrested at the corresponding threshold level, so that a certain adapted state is preserved during a subsequent growth period without phosphate supply and even appears to survive a possible turnover of proteins. When one of the proteins is occasionally replaced, the new protein will readapt to the preexisting condition and, by this mechanism, “inherit” the properties of the protein it had substituted.
The adaptive operation mode.
When a new pulse disturbs a state of least energy dissipation, the uptake system is in flux and strives for another energetically favorable state, in which its energetic and kinetic properties are conformed to the sudden increase in the external phosphate concentration according to the constraints described above. Presupposing that the cells are exposed sufficiently long to elevated phosphate levels, an adapted state attained at the beginning of the pulse is not stable, since the external concentration further decreases by the newly adapted uptake activity. In consequence, the uptake system has to readapt again to the lower external concentration. In this case the system selects the concentration level to which it conforms its properties. Which level and how fast it is selected, depends on the growth history (see Figs. 1 and 2). From what has been outlined above, we can safely assume that adaptation to a diminishing external concentration is paralleled by an adaptive interplay between the transport system and the ATP-synthase. In this interplay the adaptive adjustment in each of these two subsystems is made in view of the manifestations of the other system (for example, in a state of minimal entropy production the properties of the transport system are conformed to the selected difference between external and cytoplasmic phosphate concentration, the latter being determined by the properties of the ATP-synthase). Since the adaptive reconstruction takes a certain time, the time course of the decrease in the external concentration determines how many adaptive steps occur during a pulse and hence, which stable adapted state finally appears, when the external concentration has dropped to the threshold value again. For this reason the time course is decisive for transfer of information from one pulse to the next, when distinct adapted states resulting from diverse time courses differently affect subsequent adaptive modes. This “mechanism” explains how cyanobacteria are able of discriminating between patterns of phosphate pulses in a way that leads to nonidentical physiological responses. The here proposed model has an important community-related aspect. Only when the joint uptake activity of the whole community transforms fluctuations in the external phosphate concentration into a sequence of distinct pulses to which the individual cell is capable of adjusting its metabolism in the way described above, information about the preceding phosphate supply is transferred from one adaptive event to the next. Hence, a dialectic relation between the activities of individual organisms and the community (including the phosphate suppliers) appears to be the ultimate basis for organismal experience. For this reason information processing has a reference beyond the metabolism of a single cell. The fact that organismal experience will also take place when a single cell is exposed to a special experimental setup in which this cell is able to adapt to changing concentrations, does not disprove this conclusion. In this case the “community” consists of the experimentalist and the cell.
Information processing by interplay of two distinct manifestations of a certain physiological behavior appears to be widespread, even though the substantial basis for the observed phenomena can be completely different. In a recent publication it was shown that information about an asymmetrical treatment in Bidens seedlings is stored and can be later recalled in a context dependent way, indicating that also in this case the system is capable of interpreting stored information in one way or the other.21,22 Hence, we may conclude that information processing about environmental changes reveals in totally different systems the characteristic manifestations of elements, as proposed by system theory (see also ref. 26).
The “self-description of the system”.
The experience of environmental changes via adaptive events allows understanding how a cellular system could construct such a model of itself and/or its environment, as postulated by Rosen for anticipatory systems.23 In a previous publication Trewavas has postulated that such modelling potentially takes place on an “adaptive representational network”.18 A generalization of the adaptive properties of the phosphate uptake system to other energy converting subsystems of the cell, incessantly adapting to each other via interplay between adapted states and adaptive modes, corresponds to this idea. In this interplay the subsystems are supposed to “observe” in adaptive modes alterations of their adapted states, as described above for the interplay between transport system and ATP-synthase. In any of these subsystems a new adaptive event is initiated, when the stationary substrate concentration, to which this system previously had been adjusted in an energetically favorable manner, is disturbed by an alteration of the external milieu. In response to this disturbance the subsystem in question will then adopt new properties, leading to a new stationary state, which is characterized by altered substrate and product concentrations. But then the other energy converting subsystems of the cell are no longer conformed to the resulting product concentration and have to readapt, presupposing that the other subsystems also develop towards states of least energy dissipation. This may again affect the initially attained adapted state and lead here to a new adaptive response, and so on. As long as the subsystems are not sufficiently conformed to each other, the total system, comprising of the cellular system and its environment, is remote from a balanced condition that here functions as an attractor for the overall adaptive dynamics. We may now postulate that the whole system experiences any displacement from that balanced condition as a tension. In this case the tension will be the greater, the greater the disturbance of a subsystem by an alteration of the respective substrate concentration. If so, the tension of the cell could be considered as an internal representation of the system, since it reflects a deviation from an organism-specific idealized state, directing different adaptive modes towards the corresponding adapted states, such that the organism remains identical with itself. For theoretical reasons we expect that this process is not confined to interplay of energy converting subsystems. A reconstitution of the whole cellular system will also include a rearrangement of proteins involved in the formation of so-called functioning-dependent structures.24
It should be noted that the interpretation presented above derives a theoretical justification from Whitehead's philosophy of organisms. This philosophy departs from Newtonian paradigm and Cartesian reductionism in that it offers a nonmechanistic explanation of the relation between experience and self-constitution of an organism. Accordingly, we have adopted Whitehead's notion of an ‘actual entity’, also termed ‘actual occasion’ of experience, for the definition of an ‘adaptive event’. The integration of experienced (‘prehended’) entities into a concrete organismic unit (‘concrescence’, in Whitehead's terminology) corresponds to the transition of adaptive operation modes to adapted states. Finally, Whitehead's ‘defining characteristics’ imposed on the adaptive interplay of subsystems is assigned to an organism-specific tension free state for which the organism strives in self-sustaining recurrence in every new experience of an environmental alteration. For further-going explanations, see Whitehead, 1978, reference 25.
Acknowledgements
The authors thank Dan Danielopol for stimulating discussions. The work has been supported by the Austrian Science Fund (Project P 16237-B05). The technical assistance of Johanna Schmidt is greatfully acknowledged.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3242
References
- 1.Falkner G, Wagner F, Small JV, Falkner R. Influence of fluctuating phosphate supply on the regulation of phosphate uptake by the blue-green alga Anacystis nidulans. J Phycol. 1995;31:745–753. [Google Scholar]
- 2.Wagner F, Falkner R, Falkner G. Information about previous phosphate fluctuations is stored via an adaptive response of the high-affinity phosphate uptake system of the cyanobacterium Anacystis nidulans. Planta. 1995;197:147–155. [Google Scholar]
- 3.Falkner G, Wagner F, Falkner R. The bioenergetic coordination of a complex biological system is revealed by its adaptation to changing environmental conditions. Acta Biotheoretica. 1996;44:283–299. [Google Scholar]
- 4.Falkner R, Falkner G. Distinct adaptivity during phosphate uptake by the cyanobacterium Anabaena variabilis reflects information processing about preceding phosphate supply. J Trace Microprobe Techn. 2003;21:363–375. [Google Scholar]
- 5.Plaetzer K, Thomas SR, Falkner R, Falkner G. The microbial experience of environmental phosphate fluctuations. An essay on the possibility of putting intentions into cell biochemistry. J Theor Biol. 2005;235:540–554. doi: 10.1016/j.jtbi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Hudson JJ, Taylor WD, Schindler DW. Phosphate concentrations in lakes. Nature. 2000;406:54–56. doi: 10.1038/35017531. [DOI] [PubMed] [Google Scholar]
- 7.Droop MR. Some thoughts on nutrient limitation of algae. J Phycol. 1973;48:689–733. [Google Scholar]
- 8.Falkner G, Falkner R, Schwab AJ. Bioenergetic characterization of transient state phosphate uptake by the cyanobacterium Anacystis nidulans. Theoretical and experimental basis for a sensory mechanism adapting to varying environmental phosphate levels. Arch Microbiol. 1989;152:353–361. [Google Scholar]
- 9.Falkner G, Wagner F, Falkner R. On the relation between phosphate uptake and growth of the cyanobacterium Anacystis nidulans. CR Acad Sci Paris, Sciences de la vie/Life sciences. 1994;317:535–541. [PubMed] [Google Scholar]
- 10.Kratz WA, Myers J. Nutrition and growth of several blue-green algae. Am J Bot. 1955;42:282–287. [Google Scholar]
- 11.Thellier M. An electrokinetic interpretation of the functioning of biological systems and its application to the study of mineral salts absorption. Ann Bot. 1970;34:983–1009. [Google Scholar]
- 12.Thellier M. Nonequilibrium thermodynamics and electrokinetic Interpretation of biological systems. J theor Biol. 1971;31:389–393. doi: 10.1016/0022-5193(71)90017-8. [DOI] [PubMed] [Google Scholar]
- 13.Thellier M, Ripoll C, Vincent JC, Mikulecki D. Interpretation of the fluxes of substances exchanged by cellular systems with their external medium. In: Bonzon M, Degli Agosti R, editors. Some physicochemical and mathematical tools for understanding of living systems. Greppin University of Geneva; 1993. pp. 221–277. [Google Scholar]
- 14.Falkner G, Falkner R, Wagner F. Adaptive phosphate uptake behaviour of the cyanobacterium Anacystis nidulans: Analysis by a proportional flow-force relation. CR Acad Sci Paris, Sciences de la vie/Life sciences. 1993;316:784–787. [PubMed] [Google Scholar]
- 15.Trewavas A. Understanding the control of plant development and the role of growth substances. Aust J Plant Physiol. 1986;13:447–457. [Google Scholar]
- 16.Bateson G. Steps to an Ecology of Mind. Chicago: The University of Chicago Press; 2000. [Google Scholar]
- 17.Falkner G, Falkner R. Objectivistic views in biology: An obstacle to our understanding of self-organisation processes in aquatic ecosystems. Freshwater Biol. 2000;44:553–559. [Google Scholar]
- 18.Trewavas A. Green plants as intelligent organisms. Trends in Plant Science. 2005;10:413–419. doi: 10.1016/j.tplants.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Wagner F, Falkner G. Concomitant changes in phosphate uptake and photophosphorylation in the blue-green alga Anacystis nidulans during adaptation to phosphate deficiency. J Plant Physiol. 1992;140:163–167. [Google Scholar]
- 20.Stucki JW. The optimal efficiency and the economic degrees of coupling of oxidative phosphorylation. Eur J Biochem. 1980;109:269–283. doi: 10.1111/j.1432-1033.1980.tb04792.x. [DOI] [PubMed] [Google Scholar]
- 21.Desbiez MO, Tort M, Thellier M. Control of a symmetry-breaking process in the course of the morphogenesis of plantlets of Bidens pilosa L. Planta. 1991;184:397–402. doi: 10.1007/BF00195342. [DOI] [PubMed] [Google Scholar]
- 22.Thellier M, Le Sceller L, Norris V, Verdus MC, Ripoll C. Long-distance transport, storage and recall of morphogenic information in plants. The existence of a sort of primitive plant ‘memory’. CR Acad Sci Paris, Science de la vie/Life Sciences. 2000;323:81–91. doi: 10.1016/s0764-4469(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 23.Rosen R. Anticipatory Systems. Philosophical, mathematical and methodological foundations. Oxford: Pergamon Press; 1985. [Google Scholar]
- 24.Thellier M, Legent G, Norris V, Baron C, Ripoll C. Introduction to the concept of functioning-dependent structures in living cells. C R Biologies. 2004;327:1017–1024. doi: 10.1016/j.crvi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Whitehead AN. In: Process and Reality. An Essay in Cosmology. Corrected edition. Griffin DR, Sherburne DW, editors. London: The Free Press, Collier Macmillan Publishers; 1978. (first published in 1929 by Macmillan Publishing Co., Inc.) [Google Scholar]
- 26.Tafforeau M, Verdus MC, Norris V, Ripoll C, Thellier M. Memory processes in the response of plants to environmental signals. Plant Signaling & Behavior. 2006;1:9–14. doi: 10.4161/psb.1.1.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]