Abstract
Electrical signaling and rapid closure of the carnivorous plant Dionaea muscipula Ellis (Venus flytrap) have been attracting the attention of researchers since XIX century, but the exact mechanism of Venus flytrap closure is still unknown. We found that the electrical stimulus between a midrib and a lobe closes the Venus flytrap leaf by activating motor cells without mechanical stimulation of trigger hairs. The closing time of Venus flytrap by electrical stimulation of motor cells is 0.3 s, the same as mechanically induced closing. The mean electrical charge required for the closure of the Venus flytrap leaf is 13.6 µC. Ion channel blockers such as Ba2+, TEACl as well as uncouplers such as FCCP, 2,4-dinitrophenol and pentachlorophenol dramatically decrease the speed of the trap closing. Using an ultra-fast data acquisition system with measurements in real time, we found that the action potential in the Venus flytrap has a duration time of about 1.5 ms. Our results demonstrate that electrical stimulation can be used to study mechanisms of fast activity in motor cells of the plant kingdom.
Key Words: action potential, electrophysiology, electrical signaling, Venus flytrap, motor cells
Introduction
Since the end of the 19th century, rapid closure of the carnivorous plant Dionaea muscipula Ellis (Venus flytrap) has been attracting the attention of researchers and as a result its mechanism has been widely investigated.1–4 This small plant consists of 5–7 leaves; each leaf is divided into two parts. The upper part of the leaf has a pair of trapezoidal-shaped lobes held together by a blade or midrib. The center of each lobe contains three or more sensitive trigger hairs with a red anthocynanin pigment that attracts insects. The edge of each lobe is lined with hair-like projections or cilia. The lower part of the leaf is sometimes referred to as the footstalk.5 These six trigger hairs protruding from the upper leaf epidermis of the Venus flytrap act as mechanosensors. When an insect touches the trigger hairs, these mechanosensors generate an electrical signal that acts as an action potential, which activates the motor cells. Macfarlane6 found that two mechanical stimuli required for the trap closing should be applied within an interval from 0.75–20 s. Brown and Sharp7 found that at high temperature of 35–40°C usually only one mechanical stimulus is required.
According to Goldsworthy,8 bioelectrochemical signals that look like nerve impulses exist in all plants. The inducement of nonexcitability after excitation and the summation of subthreshold irritations were developed in the vegetative and animal kingdoms in protoplasmic structures prior to morphological differentiation of nervous tissues. These protoplasmic structures merged into the organs of a nervous system and adjusted the interfacing of the organism with the environment. Some neuromotoric components include acetylcholine neurotransmitter, cellular messenger calmodulin, cellular motors actin and myosin, voltage-gated channels, and sensors for touch, light, gravity and temperature.9,10 Although this nerve-like cellular equipment has not reached the same great complexity as in animal nerves, a simple neural network has been formed within the plasma membrane of a phloem10 or plasmodesmata11 enabling it to communicate efficiently over long distances. The reason why plants have developed pathways for electrical signal transmission most probably lies in the necessity to respond rapidly to environmental stress factors. Different environmental stimuli evoke specific responses in living cells, which have the capacity to transmit a signal to the responding region. In contrast to chemical signals such as hormones, electrical signals are able to rapidly transmit information over long distances. Electrical potentials have been measured at the tissue and whole plant level.12–14 Cl-, K+, Ca2+ and H+ are actively involved in the establishment and modulation of electrical potentials.10,12,14 Most of the plant action potentials studied so far has a velocity in the range of 0.002–40 m/s.10–17 Electrical signals can be generated at any site of the symplastic continuum by environmental stimuli such as changes in temperature, touch or injury.
Electrophysiologists have measured the generation of action potentials in Venus flytrap for the last 130 years. These measurements were taken with extremely slow registration systems and without low pass filters and with different time constants of analog voltmeters, depending on input resistance and capacitance t = RC, resulting in erroneous data.1,16–19 Due to electronic effects of aliasing,20 many publications report different amplitudes (from 10 mV to 150 mV), durations (from 100 ms to 10 s), and speeds of propagation of action potentials (from 0.03 m/s to 0.20 m/s).1,16–19,21,22 Plant physiologists correctly criticized these results.23 The slow action potentials may be the result of the Venus flytrap closure, but not its cause.23 Recently, it was shown using high-speed video under ultraviolet light that the fast closure of Venus flytrap starts at about 40 ms after mechanical stimulation and completes in 0.3–0.7 s.4
The mechanism by which Venus flytrap snaps is not clearly understood and a number of conflicting models have been proposed.1,2,4,24 What is consistent experimentally is the fact that the first step involves the generation of receptor and action potentials that induce mechanical closing of the Venus flytrap.1,2,25,26 In addition, the mechanism of the rapid mechanical trap closure is also poorly understood and has been explained either by the acid growth response, wall loosening,24 or by a loss of turgor pressure in the upper epidermis. Forterre et al explained the closure as a slow diffusion that changes the geometric parameters to a snap-buckling instability, which causes the fast reactions.4
Understanding the nature of regulatory relations of the plant organism with its environment is a basic biophysical problem. The influence of the environment has a direct bearing on the tasks of controlling the growth and development of plants.
This report investigates the electrical signaling in the carnivorous plant Venus flytrap. The key feature involves using an ultra-fast data acquisition system with measurements in real time. Using this system, we found that the action potential in the Venus flytrap is fast enough to induce the closure of the leaves by the motor cells. We also found that the electrical stimulus between a midrib and a lobe closes the Venus flytrap leaf by activating motor cells without mechanical stimulation of trigger hairs. These results demonstrate that electrical stimulation and ultra-fast data acquisition can be used to study mechanisms of fast activity in motor cells of the plant kingdom.
Materials and Methods
Data acquisition.
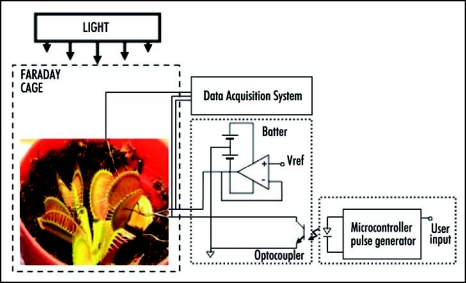
A novel real-time experimental setup using an ultra- fast data acquisition system along with a charged capacitor method for the electrical stimulation of Venus flytrap was developed (see Fig. 1). All measurements were conducted in the laboratory at constant room temperature inside a Faraday cage mounted on a vibration-stabilized table. In order to estimate possible high frequency content of the responses evoked, a high performance National Instruments data acquisition system was used. High speed data acquisition of low-pass filtered signals was performed using microcomputers with simultaneous multifunction I/O plug-in data acquisition board NI-PXI-6115 or NI-PCI-6115 (National Instruments) interfaced through a NI SCB-68 shielded connector block to 0.1 mm thick nonpolarizable reversible Ag/AgCl electrodes. The results were reproduced on a workstation with data acquisition board NI 6052E DAQ with input impedance of 100 GΩ interfaced through a NI SC-2040 Simultaneous Sample and Hold. The system integrates standard low-pass anti-aliasing filters at one half of the sampling frequency, in our case 125 KHz.
Figure 1.
Experimental setup.
The Shannon sampling theorem states that the input signal must be sampled at a rate greater than twice the highest frequency component in the signal. This critical sampling rate is called the Nyquist rate. Mathematically, fs/2 > fmax, where fs the sampling frequency and fmax is the maximum frequency of the sampled signal. Violation of the Nyquist criterion is called undersampling and results in aliasing. All data presented in this paper were collected on high-speed data acquisition system to examine the existence of any higher frequency components of action potentials.
Electrodes.
Ag/AgCl electrodes were prepared from Teflon coated silver wires.12,13,15 Following insertion of the electrodes into lobes and a midrib, the traps closed. We allowed plants to rest until the traps were completely open.
Plant electrostimulation.
We applied a novel electrostimulation method, as presented in Figure 1, to allow separate control of both amplitude and timing of the stimulation pulse and to provide a high-impedance optical isolation when the plant is not stimulated. We used a custom microcontroller device (Texas Instruments MSP430F149) to generate logic pulse of the precisely controlled duration on user's request. The pulse triggers a signal conditioning circuit with preset reference voltage through the optocoupler. This approach effectively disconnects the plant from the stimulation system when the pulse is not present. The amplifier allows for the active driving of the pulse with very low output resistance of the stimulation system. A separate battery was used to eliminate high-frequency noise of the pulse generator system.
The charge injection method has been used to precisely estimate the amount of electrical energy necessary to cause the closing of the leaves. Two critical parameters have been analyzed: the amount of charge and the applied voltage. Both parameters were tested to determine the minimum amount of charge and the minimum voltage sufficient to close the plant's trap. A double pole, double throw (DPDT) switch was used to connect the known capacitor to the voltage source during charging, and then to the plant during plant stimulation. Since the charge of capacitor C connected to the voltage source V is Q = CV we can precisely regulate the amount of charge using different capacitors and applying various voltages. By changing switch position, we can connect the charged capacitor to the plant and induce evoked response.
Images.
Digital video camera recorders Sony DCR-HC36 and Canon ZR300 were used for the monitoring of Venus flytraps and to collect digital images, which were analyzed frame by frame. The NTSC format consists of 29.97 interlaced frames of video per second.
Chemicals.
Carbonylcyanide-4-trifluoromethoxyphenyl hydrazone (FCCP), 2,4-dinitrophenol (DNP), 2,3,4,5,6-pentachlorophenol (PCP), gelatin, ZnCl2, BaCl2 and tetraethylammonium chloride (TEACl) were obtained from Fluka (New York, NY).
Plants.
One hundred bulbs of Dionaea muscipula (Venus flytrap) were purchased for this experimental work from Fly-Trap Farm (Supply, North Carolina) and grown in a well drained peat moss in plastic pots at 22°C with a 12:12 hr light:dark photoperiod. The soil was treated with distilled water. All experiments were performed on healthy adult specimens. Plants were fed a 6 mm × 6 mm × 2 mm cube of 4 % (w/v) gelatin and induced to close by stimulating two of the three trigger hairs.
Results and Discussion
As a control experiment, we first recorded the electrical signals induced by mechanical stimulation of trigger hairs. Then, we tested the role of electrical signaling by generating similar electrical pulses using a custom developed electrical stimulator. We discovered that artificial electrical stimulation induces the closing of the trap with the same speed as in vivo mechanical stimulation.
Mechanostimulation.
Plants can perceive mechanical stimuli. This process involves mechanosensitive channels that are found in all types of cells, from animal and plant cells to fungi and bacteria. These channels are ideal transducers of physiologically relevant mechanical forces and are involved in the growth, development, and response to environmental stress in higher plants. Mechanosensory ion channels in plants are activated by mechanical stress and then this information was transduced into electrical signals. Detailed analyses of the electrophysiology in higher plants are difficult because such plants are composed of complex tissues.
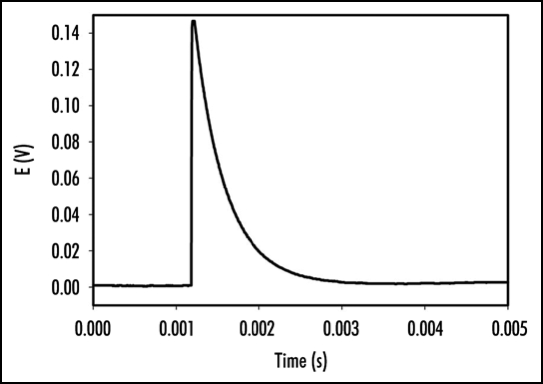
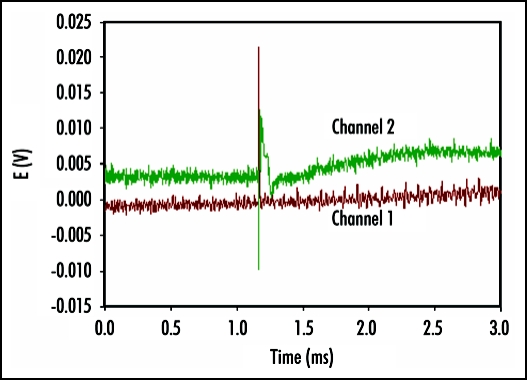
Perception and response to mechanical stimuli are essential at the cellular and organismal levels. Venus flytrap can be closed by a mechanical stimulation of trigger hairs using a cotton thread or by a small piece of gelatin. Mechanically stimulated closing of Venus flytrap is characterized by a slow initial phase, a rapid intermediate and slow final phase. It has been reported that a mechanical stimulation of trigger hairs induces action potentials in the upper leaf of the Venus flytrap plant.1,3,11,18,19,21–28 The measurements of the Venus flytrap's action potential have been the subject of research for many scientists ever since the pioneering works of Burdon-Sanderson.1,18,19 The novelty of the present paper is to use a high-speed data acquisition system that allows resolving the action potential's duration and amplitude. The electrical signal, induced by mechanical stimulation of a single trigger hair of Venus flytrap by a small piece of gelatin, resembles an action potential with duration time of 1.5 ms (Fig. 2). Action potential propagates from mechanosensitive trigger hairs of the lobe to the midrib in the upper part of the leaf, as presented in (Fig. 2). It features a sharp spike, followed by a more gradual return to the original resting state. Electrical signaling was also recorded between the midrib or lobe and the lower part of a leaf. However, in control experiments, we inserted two pairs of Ag/AgCl electrodes in the footstalk and on both channels, no action potential associated with electrical or mechanical stimulation of Venus flytrap closure was observed. This indicates that action potential signaling is limited to the upper part of the leaf. A few minutes after Venus flytrap closing, electrical signaling was, however, detected in the lower part of the leaf of Venus flytrap (Fig. 3) in the form of graded potentials with amplitudes of 20 mV or less. A graded potential is a wave of electrical excitation that appears as a result of short lived depolarization or hyperpolarization of an area of the plasma membrane in conductive bundles of plants. This phenomenon causes local flows of electrical current that decrease with distance. Graded potentials get weaker as they travel along nerve fibers or conductive bundles in plants, whereas action potentials remain the same strength as they travel.
Figure 2.
Action potential induced in Venus flytrap by a peace of gelatin stimulating a single trigger hair. One Ag/AgCl electrode (+) was located in the midrib and second Ag/AgCl electrode (-) was in the center of lobe. The frequency of scanning was 250,000 samples per second.
Figure 3.
Electrical signaling in the lower part of the leaf of Venus Flytrap two minutes after closing of upper leaf induced by a peace of gelatin. Distance between electrodes on each channel was 1 cm, distance between two channels was 2 cm. The frequency of scanning was 200,000 samples per second.
Electrostimulation.
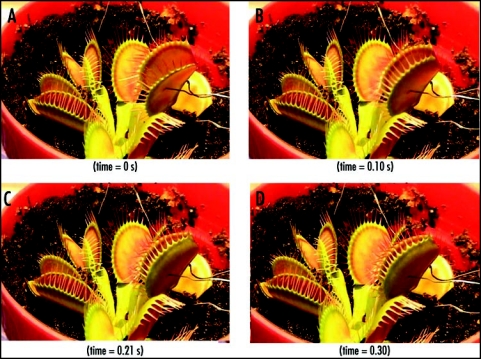
Using our new stimulation system, it was evident that the application of an electrical stimulus between the midrib (positive potential) and a lobe (negative potential) causes Venus flytrap to close the trap without any mechanical stimulation. Figure 4 demonstrates the closing of the Venus flytrap in 0.3 s after electrical stimulation. The average stimulation pulse voltage sufficient for rapid closure of the Venus flytrap was 1.50 V (standard deviation is 0.01 V, n = 50) for 1 s. The inverted polarity pulse with negative voltage applied to the midrib could not close the plant. We were unable to open the plant by applying impulses in the same voltage range with different polarities for pulses of up to 100 s. It was found that energy for trap closure is generated by ATP hydrolysis.29 ATP is used by the motor cells for a fast transport of protons. The amount of ATP drops from 950 µM per midrib before mechanical stimulation to 650 µM per midrib after stimulation and closure.29 However, it is not clear if electrical stimulation triggers closing process in the motor cells, or contributes energy to the closing action.
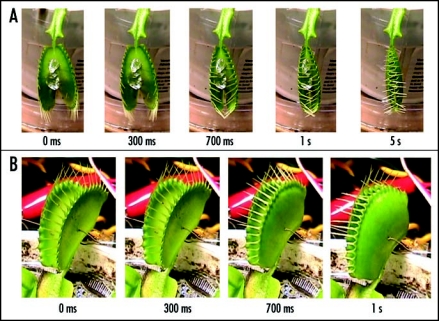
Figure 4.
Sequence of Venus flytrap photos before (A) and during (B–D) the trap closure by electrical stimulation. Ag/AgCl electrodes are located in a midrib and in the lobe. These results were reproduced 50 times on different Venus flytrap plants.
Charge induced closing.
We hypothesized that the action potential delivers sufficient electrical charge to the midrib, which can activate the motor cells. To check this hypothesis, we measured effects of transmitted charge from the charged capacitors between the lobe and the midrib of Venus flytrap. Transmission of a single electrical charge (mean 13.63 µC, median 14.00 µC, std. dev. 1.51 µC, n = 41) causes closure of a trap and induces an electrical signal propagating between the lobes and the midrib. This electrical signal in lobes was not an action potential, because its amplitude depended on the applied voltage from the charged capacitor. Charge induced closing of a trap plant can be repeated 2–3 times on the same Venus flytrap plant after reopening.
Repeated application of smaller charges demonstrates a summation of stimuli. If we apply two or more injections of electrical charges within a period of less then 20 s, the Venus flytrap upper leaf closes as soon as the total of 14 µC charge is transmitted. Similar phenomenon was reported by Czaja,30 who determined that the intensity of threshold stimuli to be 2.4 µC for a closing electrostimulation of another carnivorous plant Aldrovanda vesiculosa, and 0.91 µC for an opening electrostimulation. Our attempts to open the Venus flytrap upper leaf by changing polarity of injected charge and increasing the charge from 14 µC to 100 µC were not successful. Usually, the trap opens a few days after closing in the same way as after mechanically stimulated closing.
Previous work by Brown and Sharp7 indicated that electrical shock between lower and upper leaves can cause the Venus flytrap to close, but in their article, the amplitude and polarity of applied voltage, charge, and electrical current were not reported. The trap did not close when we applied the same electrostimulation between the upper and lower leaves as we applied between a midrib and a lobe, even when the injected charge was increased from 14 µC to 750 µC. It is probable that the electroshock induced by Brown and Sharp7 had a very high voltage or electrical current.
Effects of ion channel blockers.
According to literature, the amplitudes of action and resting potentials in the Venus flytrap depend on the concentration of K+ and Ca2+ cations.3 Glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid and LaCl3 completely inhibit the excitability of Venus flytrap,3 which indicates that the ion channels are responsible for the propagation of action potentials. Electrical signals can propagate via plasmodesmata to other cells of the symplast.11 An action potential is evoked when the stimulus is strong enough to depolarize the membrane. Subsequently, the action potential, characterized by a large transient depolarization, allows the rapid transmission of information via plasmodesmata or conductive bundles.
In terms of the electrophysiology of the Venus flytrap, responses are considered to be in three stages: (i) stimulus perception, (ii) signal transmission and (iii) induction of response. In the Venus flytrap, the first stage is due to the receptor potential,31 a transient depolarization with a critical threshold that triggers action potentials, which in turn is responsible for stages (ii) and (iii). Receptor potentials are generated by mechanosensitive ion channels.32 Like the action potential, a critical threshold depolarization can activate Ca2+-permeable Cl- channels and K+ channels that may result in turgor regulation. However, since higher plants are composed of complex tissues, detailed analysis of electrical phenomena is rather difficult to attain, and as a result the mechanism for generating the receptor potential has not been established.
It is most probable that rapidly activating anion channels33 as well as K+ channels are involved in the propagation of action potentials in plants. TEACl is known to be a blocker of potassium channels in plants. We found that 10 mM aqueous solution of tetraethylammonium chloride (TEACl) inhibits the trap closure of Dionaea muscipula Ellis which was induced by either mechanical (Fig. 5A) or electrical stimulation (Fig. 5B). Blockers of plant anion channels33 and Ca2+-permeable channels, Ba2+ and Zn2+ dramatically decrease the speed of a trap closure induced by a gelatin (Fig. 6A) or electrical stimulus (Fig. 6B).
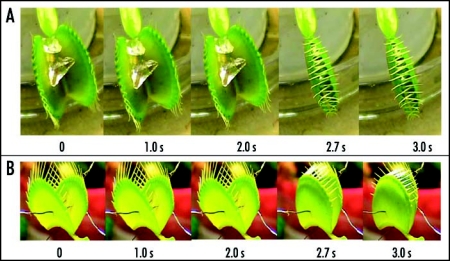
Figure 5.
Sequence of Venus flytrap photos after stimulation of trigger hairs by a small piece of a gelatin (A) or by electrical stimulation (B). 50 mL of 10 mM TEACl was added to soil 55 hours before experiments. These results were reproduced 14 times on different Venus flytrap plants.
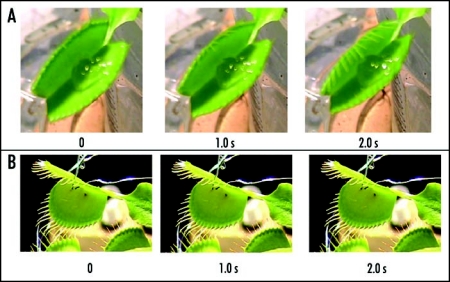
Figure 6.
Sequence of Venus flytrap photos after stimulation of trigger hairs by a small piece of a gelatin (A) or by electrical stimulation (B). Fifty milliliters of 5 mM BaCl2 was added to soil 55 hours before experiments. These results were reproduced seven times on different Venus flytrap plants.
Effects of protonophoric uncouplers.
The rapid closure of Venus flytrap involves cell enlargement, which can be initiated by acidifying the cell walls to pH 4.5 and below.24 Leaves infiltrated with neutral buffers that keep pH above 4.5 do not close in response to stimulation of their trigger hairs even though action potentials are generated. It is known that ATP is used by the motor cells for a fast transport of protons.24,29 Rea34 studied the dynamics of protons efflux from the trap lobes of Venus flytrap mediated by a K+-H+ exchange mechanism during digestion.
Pentachlorophenol, FCCP and 2,4-dinitrophenol, compounds to be known as protonophoric uncouplers, are effective inhibitors of the trap closure (Figs. 7 and 8). They inhibit both mechanically (Fig. 7A) and electrically (Figs. 7B, 8A and B) induced trap closure. Protonophores, which are soluble in both water and lipids, permeate the lipid phase of a membrane by diffusion and transfer protons across the membrane, thus eliminating a proton concentration gradient and/or a membrane potential. Hodick and Sievers25 reported an excitability inhibition of a Dionaea leaf mesophyll cells using 2,4-dinitrophenol. Resting potential and excitability are completely restored after 30 min of washing a standard medium. This explains the results shown in Figure 7A involving the inhibition of mechanically induced trap closure. Inhibition of electrically induced trap closure in the presence of protonophores (Figs. 7B, 8A and B) when electrical charge is submitted to a midrib and motor cells, can be caused by dissipation of a proton gradient during ATP hydrolysis in motor cells and depolarization of a membrane.
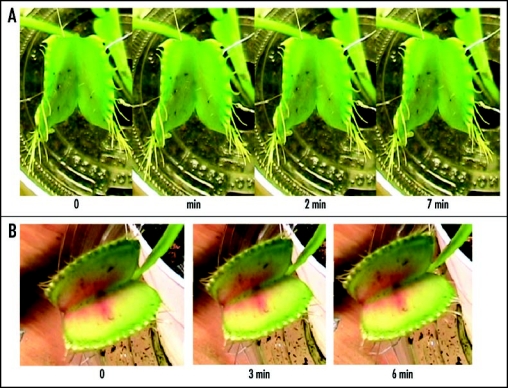
Figure 7.
Sequence of Venus flytrap photos after stimulation of trigger hairs by a small piece of a gelatin (A) or by electrical stimulation (B). Fifty milliliters of 0.1 mM pentachlorophenol was added to soil 48 hours before experiments. These results were reproduced five times on different Venus flytrap plants.
Figure 8.
Sequence of Venus flytrap photos after electrical stimulation. (A) Fifty milliliters of 0.5 mM 2,4-dinitrophenol was added to soil 48 hours before experiments. (B) Fifty milliliters of 10 µM FCCP was added to soil 48 hours before experiments. These results were reproduced seven times on different Venus flytrap plants.
Uncouplers are generally weak acids, and are often used to inhibit photosynthetic water oxidation. This phenomenon is due to their ability to be oxidized by the manganese cluster of the O2-evolving complex of photosystem II (PSII) and chloroplast.14,35 These oxidized uncouplers can be reduced by the membrane pool of plastoquinone, leading to formation of an artificial cyclic electron transfer chain around PSII involving uncouplers as redox carriers. Protonophores such as 2,3,4,5,6-pentachlorophenol (PCP), and 4,5,6,7-tetrachloro-2-trifluoromethylbenzimidazole (TTFB) inhibit the Hill reaction with K3Fe(CN)6 in chloroplasts. They also uncouple electron transport, accelerate the deactivation of the S-2 and S-3 states on the donor side, and facilitate the oxidation of cytochrome b559 on the acceptor side of PSII.14,35
Green plants are a unique canvas for studying signal transduction. They provide the foundation for discovering and improving biosensors, and are essential to the development of alternative sources of energy. All processes of life generate electric fields in every organism. The conduction of electrochemical excitation is regarded as a universal property of living organisms and arose in connection with a need for the transmission of information in response to an external influence from one part of a biological system to another.36 The electrical stimulation technique can be used to study mechanisms of fast signaling and activity in motor cells of the plant kingdom. Employing this nascent computerized technology will provide opportunities for the detection of fast electrical signaling in green plants in real time.
Acknowledgements
This work was partially supported by grants from the National Science Foundation grant DMR-0521611 and NASA grant NAG81888.
Abbreviations
- FCCP
carbonylcyanide-4-trifluoromethoxyphenyl hydrazone
- DNP
2,4-dinitrophenol
- PCP
2,3,4,5,6-pentachlorophenol
- TEACl
tetraethylammonium chloride
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=4217
References
- 1.Burdon-Sanderson J. Note on the electrical phenomena which accompany stimulation of the leaf of Dionaea muscipula Ellis. Phil Proc R Soc Lond. 1873;21:495–496. [Google Scholar]
- 2.Darwin C. Insectivorous Plants. London: Murray; 1875. [Google Scholar]
- 3.Hodick D, Sievers A. The influence of Ca2+ on the action potential in mesophyll cells of Dionaea muscipula Ellis. Protoplasma. 1989;133:83–84. [Google Scholar]
- 4.Forterre Y, Skothelm JM, Dumals J, Mahadevan L. How the Venus flytrap snaps. Nature. 2005;433:421–425. doi: 10.1038/nature03185. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd FE. The Carnivorous Plants. New York: Ronald; 1942. [Google Scholar]
- 6.Macfarlane JM. Contributions to the history of Dionaea muscipula Ellis. Contrib Bot Lab Penna. 1892;1:7–44. [Google Scholar]
- 7.Brown WH, Sharp LW. The closing response in Dionaea. Bot Gaz. 1910;49:290–302. [Google Scholar]
- 8.Goldsworthy A. The evolution of plant action potentials. J theor Biol. 1983;103:645–648. [Google Scholar]
- 9.Roshchina VV. Neurotransmitters in Plant Life. Enfield: Science Publ.; 2001. [Google Scholar]
- 10.Volkov AG, editor. Plant Electrophysiology. Berlin: Springer; 2006. [Google Scholar]
- 11.Van Bel AJE, Ehlers K. Electrical signaling via plasmodesmata. In: Oparka KJ, editor. Plasmodesmata. Oxford: Blackwell; 2005. pp. 263–278. [Google Scholar]
- 12.Volkov AG. Green plants: Electrochemical interfaces. J Electroanal Chem. 2000;483:150–156. [Google Scholar]
- 13.Volkov AG, Haack RA. Insect induces bioelectrochemical signals in potato plants. Bioelectrochem Bioenerg. 1995;35:55–60. [Google Scholar]
- 14.Volkov AG, Brown CL. Electrochemistry of plant life. In: Volkov AG, editor. Plant Electrophysiology—Theory and Methods. Berlin: Springer; 2006. pp. 437–459. [Google Scholar]
- 15.Ksenzhek OS, Volkov AG. Plant Energetics. San Diego: Academic Press; 1998. [Google Scholar]
- 16.Sibaoka T. Action potentials in plant organs. Symposia Soc Experim Biol. 1966;20:49–74. [PubMed] [Google Scholar]
- 17.Sibaoka T. Physiology of rapid movements in higher plants. Annu Rev Plant Physiol. 1969;20:165–184. [Google Scholar]
- 18.Burdon-Sanderson J, Page FJM. On the mechanical effects and on the electrical disturbance consequent on excitation of the leaf of Dionaea muscipula. Phil Proc R Soc Lond. 1876;25:411–434. [Google Scholar]
- 19.Burdon-Sanderson J. On the electromotive properties of the leaf of Dionaea in the excited and unexcited states. Phil Trans R Soc Lond. 1882;173:1–55. [Google Scholar]
- 20.Volkov AG, Ranatunga DRA. Plants as environmental biosensors. Plant Signal Behavior. 2006;1:105–105. doi: 10.4161/psb.1.3.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuhlman O, Darden E. The action potential obtained from Venus's-Flytrap. Science. 1950;111:491–492. doi: 10.1126/science.111.2888.491. [DOI] [PubMed] [Google Scholar]
- 22.DiPalma JR, McMichael R, DiPalma M. Touch receptor of Venus flytrap, Dionaea muscipula. Science. 1966;152:539–540. doi: 10.1126/science.152.3721.539. [DOI] [PubMed] [Google Scholar]
- 23.Sachs J. Lectures on the Physiology of Plants. Oxford: Clarendon Press; 1887. [Google Scholar]
- 24.Williams SE, Bennet AB. Leaf closure in the Venus flytrap: An acid growth response. Science. 1982;218:1120–1121. doi: 10.1126/science.218.4577.1120. [DOI] [PubMed] [Google Scholar]
- 25.Hodick D, Sievers A. The action potential of Dionaea muscipula Ellis. Planta. 1988;174:8–18. doi: 10.1007/BF00394867. [DOI] [PubMed] [Google Scholar]
- 26.Hodick D, Sievers A. The influence of Ca2+ on the action potential in mesophyll cells of Dionaea muscipula Ellis. Protoplasma. 1986;133:83–84. [Google Scholar]
- 27.Brown WH. The mechanism of movement and the duration of the effect of stimulation in the leaves of Dionaea. Amer J Bot. 1916;3:68–90. [Google Scholar]
- 28.Jacobson SL. The effect of ionic environment on the response of the sensory hair of Venus's flytrap. Can J Bot. 1974;52:1293–1302. [Google Scholar]
- 29.Jaffe MJ. The role of ATP in mechanically stimulated rapid closure of the Venus's flytrap. Plant Physiol. 1973;51:17–18. doi: 10.1104/pp.51.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czaja ATH. Reizphysiologische Untersuchungen an Aldrovandia vesiculosa L. Arch Gesamte Physiologie Menschen Tiere. 1924;206:635–658. (Ger). [Google Scholar]
- 31.Benolken RM, Jacobson SL. Response properties of a sensory hair excised from Venus's flytrap. J Gen Physiol. 1970;56:64–82. doi: 10.1085/jgp.56.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braam J. In touch: Plant responses to mechanical stimuli. New Phytologist. 2005;165:373–389. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 33.Roberts SK. Plasma membrane anion channels in higher plants and their putative functions in root. New Phytologist. 2006;269:647–666. doi: 10.1111/j.1469-8137.2006.01639.x. [DOI] [PubMed] [Google Scholar]
- 34.Rea PA. The dynamics of H+ efflux from the trap lobes of Dionaea muscipula Ellis (Venus's flytrap) Plant Cell Environm. 1983;6:125–134. [Google Scholar]
- 35.Volkov AG, Brown CL. Nanodevices in nature. In: Kumar CSSR, editor. Nanodevices for Life Sciences. Weinheim: Wiley-VCH; 2006. pp. 440–463. [Google Scholar]
- 36.Volkov AG. Electrophysiology and phototropism. In: Balushka F, Manusco S, Volkman D, editors. Communication in Plants. Neuronal Aspects of Plant Life. Berlin: Springer; 2006. pp. 351–367. [Google Scholar]