Abstract
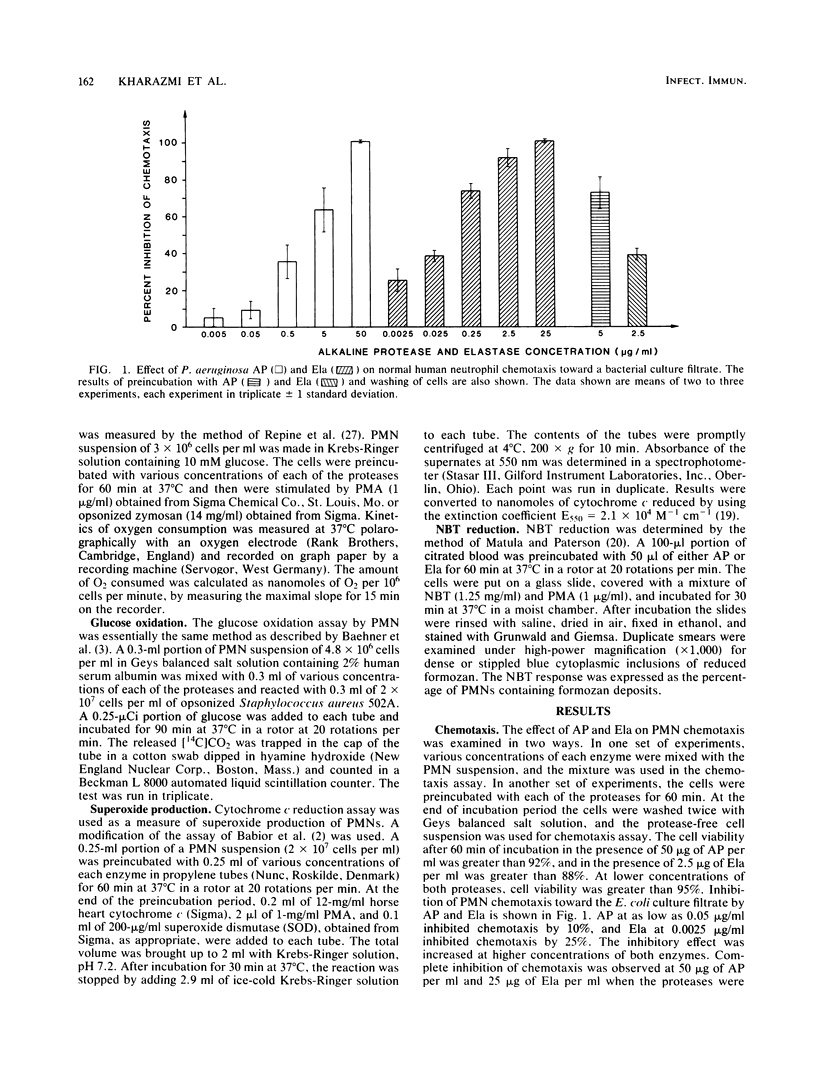
Little is known about the interaction of Pseudomonas aeruginosa extracellular products and human polymorphonuclear leukocytes. The present study was designed to examine the effect of alkaline protease and elastase purified from P. aeruginosa on human neutrophil function. Neutrophil chemotaxis, oxygen consumption, glucose oxidation, superoxide production, and nitro blue tetrazolium reduction were studied. It was found that alkaline protease and elastase at fairly low concentrations (0.05 and 0.0025 micrograms/ml, respectively) inhibited chemotaxis. The inhibitory effect of both enzymes was increased at higher concentrations. The chemotaxis of preincubated and washed cells was also inhibited. Alkaline protease but not elastase inhibited opsonized zymosan-stimulated neutrophil oxygen consumption, whereas neither of the enzymes had any effect on glucose oxidation and nitro blue tetrazolium-reducing activity of stimulated neutrophils. The data on superoxide production ability of the cells indicated that the cells preincubated with enzyme and washed were capable of producing superoxide equal to the amount produced by untreated cells when they were stimulated with phorbol myristate acetate or zymosan. However, when elastase was present in the reaction mixture, the reduction of cytochrome c as a measure of superoxide production was inhibited. Inhibition of neutrophil function, particularly chemotaxis, will have important bearing on the escape of the microorganism from the phagocytic defense system of the host. The role of these products in localized infections and avascular areas such as skin burns, cornea, and, at least initially, in chronic lung colonization in cystic fibrosis patients becomes important.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. R., Amirault H. J. Important variables in granulocyte chemiluminescence. Proc Soc Exp Biol Med. 1979 Oct;162(1):139–145. doi: 10.3181/00379727-162-40633. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G., Karnovsky M. L. Correction of metabolic deficiencies in the leukocytes of patients with chronic granulomatous disease. J Clin Invest. 1970 May;49(5):865–870. doi: 10.1172/JCI106305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood L. L., Stone R. M., Iglewski B. H., Pennington J. E. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect Immun. 1983 Jan;39(1):198–201. doi: 10.1128/iai.39.1.198-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Wallis S. N., Griffiths E. The effect of antipolymorphonuclear leucocyte serum on Pseudomonas aeruginosa infection in rabbits. Immunology. 1976 May;30(5):603–610. [PMC free article] [PubMed] [Google Scholar]
- Carney S. A., Dyster R. E., Jones R. J. The invasion of burned skin by Pseudomonas aeruginosa. Br J Dermatol. 1973 Jun;88(6):539–545. doi: 10.1111/j.1365-2133.1973.tb08016.x. [DOI] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K., Flehmig B., Høiby N., Hofmann A. Proteases of Pseudomonas aeruginosa in patients with cystic fibrosis. J Infect Dis. 1983 Apr;147(4):744–750. doi: 10.1093/infdis/147.4.744. [DOI] [PubMed] [Google Scholar]
- Gray L., Kreger A. Microscopic characterization of rabbit lung damage produced by Pseudomonas aeruginosa proteases. Infect Immun. 1979 Jan;23(1):150–159. doi: 10.1128/iai.23.1.150-159.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J Clin Invest. 1975 Oct;56(4):1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holby N., Olling S. Pseudomonas aeruginosa infection in cystic fibrosis. Bactericidal effect of serum from normal individuals and patients with cystic fibrosis on P. aeruginosa strains from patients with cystic fibrosis or other diseases. Acta Pathol Microbiol Scand C. 1977 Apr;85(2):107–114. [PubMed] [Google Scholar]
- Holder I. A., Haidaris C. G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: extracellular protease and elastase as in vivo virulence factors. Can J Microbiol. 1979 May;25(5):593–599. doi: 10.1139/m79-085. [DOI] [PubMed] [Google Scholar]
- Høiby N., Rosendal K. Epidemiology of Pseudomonas aeruginosa infection in patients treated at a cystic fibrosis centre. Acta Pathol Microbiol Scand B. 1980 Jun;88(3):125–131. doi: 10.1111/j.1699-0463.1980.tb02617.x. [DOI] [PubMed] [Google Scholar]
- Jagger K. S., Bahner D. R., Warren R. L. Protease phenotypes of Pseudomonas aeruginosa isolated from patients with cystic fibrosis. J Clin Microbiol. 1983 Jan;17(1):55–59. doi: 10.1128/jcm.17.1.55-59.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. J., Dyster R. E. The role of polymorphonuclear leucocytes in protecting mice vaccinated against Pseudomon as aeruginosa infections. Br J Exp Pathol. 1973 Aug;54(4):416–421. [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A., Valerius N. H., Høiby N. Effect of antimalarial drugs on human neutrophil chemotaxis in vitro. Acta Pathol Microbiol Immunol Scand C. 1983 Aug;91(4):293–298. [PubMed] [Google Scholar]
- Leake E. S., Wright M. J., Kreger A. S. In vitro effect of purified proteases of Pseudomonas aeruginosa on rabbit lung macrophages. Exp Mol Pathol. 1978 Oct;29(2):241–252. doi: 10.1016/0014-4800(78)90042-4. [DOI] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Matula G., Paterson P. Y. Spontaneous in vitro reduction of nitroblue tetrazolium by neutrophils of adult patients with bacterial infection. N Engl J Med. 1971 Aug 5;285(6):311–317. doi: 10.1056/NEJM197108052850603. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Oda K. Protease and elastase of Pseudomonas aeruginosa: inactivation of human plasma alpha 1-proteinase inhibitor. Infect Immun. 1979 Apr;24(1):188–193. doi: 10.1128/iai.24.1.188-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino N., Powers J. C. Pseudomonas aeruginosa elastase. Development of a new substrate, inhibitors, and an affinity ligand. J Biol Chem. 1980 Apr 25;255(8):3482–3486. [PubMed] [Google Scholar]
- Pavlovskis O. R., Wretlind B. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect Immun. 1979 Apr;24(1):181–187. doi: 10.1128/iai.24.1.181-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Ehrie M. G. Pathogenesis of Pseudomonas aeruginosa pneumonia during immunosuppression. J Infect Dis. 1978 Jun;137(6):764–774. doi: 10.1093/infdis/137.6.764. [DOI] [PubMed] [Google Scholar]
- Pennington J. E., Reynolds H. Y., Carbone P. P. Pseudomonas pneumonia. A retrospective study of 36 cases. Am J Med. 1973 Aug;55(2):155–160. doi: 10.1016/0002-9343(73)90163-0. [DOI] [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. The influence of phorbol myristate acetate on oxygen consumption by polymorphonuclear leukocytes. J Lab Clin Med. 1974 Jun;83(6):911–920. [PubMed] [Google Scholar]
- Scharmann W. Cytotoxic effects of leukocidin from Pseudomonas aeruginosa on polymorphonuclear leukocytes from cattle. Infect Immun. 1976 Mar;13(3):836–843. doi: 10.1128/iai.13.3.836-843.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmann W., Jacob F., Porstendörfer J. The cytotoxic action of leucocidan from Pseudomonas aeruginosa on human polymorphonuclear leucocytes. J Gen Microbiol. 1976 Apr;93(2):303–308. doi: 10.1099/00221287-93-2-303. [DOI] [PubMed] [Google Scholar]
- Schultz D. R., Miller K. D. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun. 1974 Jul;10(1):128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Holder I. A., Leppla S. A., Saelinger C. B. Role of exotoxin and protease as possible virulence factors in experimental infections with Pseudomonas aeruginosa. Infect Immun. 1978 Mar;19(3):839–845. doi: 10.1128/iai.19.3.839-845.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B., Nickol M. M., Jagger K. S., Saelinger C. B. Interaction of pseudomonas exoproducts with phagocytic cells. Can J Microbiol. 1982 Jun;28(6):679–685. doi: 10.1139/m82-102. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Cryz S. J., Friedman R. L., Iglewski B. H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982 Jun;36(3):1223–1228. doi: 10.1128/iai.36.3.1223-1228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


