Abstract
Leaf pavement cell expansion in light depends on apoplastic acidification by a plasma membrane proton-pumping ATPase, modifying cell wall extensibility and providing the driving force for uptake of osmotically active solutes generating turgor. This paper shows that the plant hormone ABA inhibits light-induced leaf disk growth as well as the blue light-induced pavement cell growth in pea (Pisum sativum L.). In the phytochrome chromophore-deficient mutant pcd2, the effect of ABA on the blue light-induced apoplastic acidification response, which exhibits a high fluence phase via phytochrome and a low fluence phase via an unknown blue light receptor, is still present, indicating an interaction of ABA with the blue light receptor pathway. Furthermore, it is shown that ABA inhibits the blue light-induced apoplastic acidification reversibly. These results indicate that the effect of ABA on apoplastic acidification can provide a mechanism for short term, reversible adjustment of leaf growth rate to environmental change.
Key Words: ABA, apoplastic acidification, blue light, epidermal pavement cell growth, leaf growth, pea (Pisum sativum L.), signal integration
Introduction
Plants, being sessile organisms, need to respond and acclimate to a multitude of environmental factors in order to optimize their performance. As light is the only energy source for plants, this is probably the reason for the evolution of the remarkably responsive ways by which plants are capable of adjusting physiology, development and metabolism to changing light conditions. Recently, several reviews focused on the light-regulated signal transduction networks that consist of different photoreceptors and their interactions (for example, refs. 1–3), the signaling components2,4 and their transcriptional regulation.5 Although light regulates plant processes on many levels, light-stimulated leaf growth, as part of the shade avoidance response, is a quick response6 leading to biophysical changes, which are not under extensive transcriptional regulation.5
Leaf expansion is presumably driven by the mesophyll layer and restricted by the epidermal layer.7–9 Epidermal cells can, in response to for instance changing light conditions, modify the restriction and thus can be considered to control leaf expansion. The light-stimulated expansion of epidermal pavement cells is preceded by an acidification of the cell wall compartment. Apoplastic acidification plays a central role in the control of biophysical growth parameters.7,10 Acidification is a prerequisite for growth as it increases cell wall extensibility and the uptake of solutes, necessary to allow expansion and maintain turgor, respectively.11,12 Red light, via phytochrome, but also blue light (BL), via a separate BL receptor, stimulates apoplastic acidification.6,13–15 In this study we focus on the BL-induced apoplastic acidification. BL pulses induce a transient acidification, maximal at about 4–6 min after illumination (ref. 16 and Fig. 1). A kinetic analysis of this blue light response in pea showed two distinct phases; a high-fluence (HF) response, which is phytochrome-dependent, and a low-fluence (LF) response, which is phytochrome-independent.16 Phytochrome has been shown to interact directly with the BL receptor cryptochrome in vitro in Arabidopsis.17 This agrees with the kinetic analysis of the BL acidification response in pea, where activation of phytochrome modulates the BL response.16 The HF response is characterized by signaling via calmodulin and presumably cytoplasmic calcium, whereas the LF response is independent of calmodulin.18 In the phytochrome-deficient mutant of pea (pcd2) this HF response to BL is lost.16
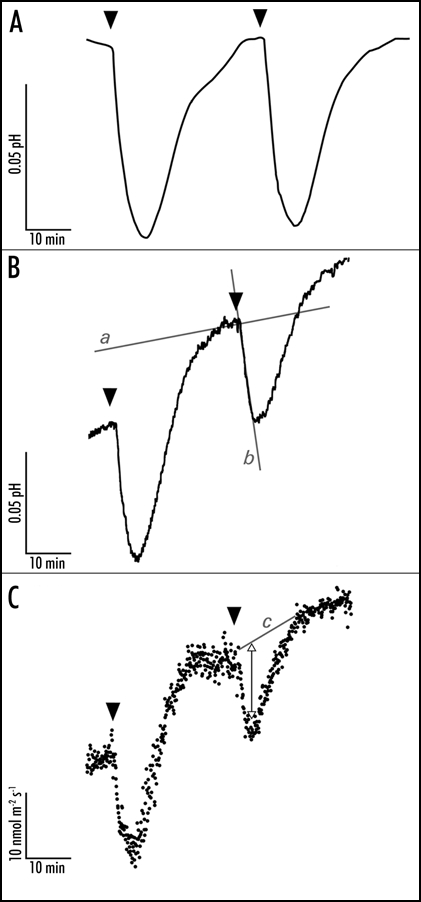
Figure 1.
Representative traces of the blue light-induced apoplastic responses at the mesophyll-facing side of the abaxial epidermal layer of a young expanding pea leaf. The apoplastic acidification is measured with a conventional flat-tipped pH electrode (A) which is carefully placed against the epidermal layer and a vibrating probe system MIFE that measures apoplastic pH (B) and proton fluxes (C) in the unstirred layer of the epidermis. After a 30s 100 µmol m−2 s−1 blue light pulse (indicated by the arrowheads) the pH lowers and the proton efflux increases within two minutes. After half an hour the pH and proton fluxes are back at basal level and a new blue light pulse is given. In all the traces it is evident that blue light affects the apoplastic pH with comparable response kinetics. The initial apoplastic acidification rate is used as the measure to investigate effects on the apoplastic pH. Hence, changes in proton fluxes (C) lead to changes in apoplastic acidification (A and B). The apoplastic acidification rate is calculated by subtracting the basal acidification rate (line a) from the blue light-induced acidification rate (line b). The change in efflux is determined by eye: basal level is drawn (line c, C) and the maximal change in flux is measured (double arrow). The trace in (A) is corrected for first order function, which describes the basic apoplastic acidification rate.
Optimizing light interception is not the only constraint in leaf development. For instance, while the necessity of capturing photons would be improved by expanding the leaf surface area, a concomitant water shortage would be mitigated by a reduction of the evaporating surface. A common observation is that drought indeed leads to a reduction of water loss through inhibition of leaf growth. Cell growth is very responsive to a lowered water potential, even more sensitive than for instance the closing response of stomata.19,20 For a long time it was assumed that drought would lead to a (small) decrease in cell turgor resulting in the inhibition of cell expansion. Later, it was found that without severe drought stress the plant hormone ABA induced a reduction of leaf growth.21,22 The effect of ABA on BL-induced leaf growth can be regarded as an example of how plants integrate contradictory signals in a specific plant process.
To investigate the integration of ABA in the signal transduction pathway of blue light-induced leaf growth, the effect of ABA on the blue light-induced acidification in epidermal strips was investigated. By using undamaged epidermal strips from the leaves of the Argenteum mutant of pea (Pisum sativum L.), lacking a component of the middle lamella resulting in a loosely attached epidermis, cell expansion can be studied without the interference of the mesophyll.23
In this study we show that ABA does affect leaf expansion at the epidermal cell level. Furthermore, we show that the proton fluxes and apoplastic acidification, as determined non-invasively by utilizing the vibrating probe technique MIFE® (microelectrode ion flux estimation;24), are inhibited by ABA, which is consistent with a direct interference of ABA with the light signaling network.
Material and Methods
Plant material.
Seeds of the Argenteum mutant of Pisum sativum L. (obtained from the Plant Genetic Resources Unit, USDA/ARS, NYS Agricultural Experimental Station Geneva, NY, USA) and the double mutant Argenteum and Phytochrome Chromophore Deficient (Arg × pcd2)8 were germinated on humid vermiculite. After seven days, the seedlings were placed on soil and grown under white light at a fluence rate of 225–250 µmol m−2s−1, provided by Philips TLD 58W reflex [cool white (840) and warm white (830)] (Eindhoven, The Netherlands) fluorescent tubes, for 15 hr light: 9 hr dark at 21°C and a relative humidity higher than 70%. Plants were watered daily and once a week the plants were given 25% Hoagland solution.
Elzenga et al.16 showed that pleiotropic effects of the pcd2 mutation on the composition of other pigments are not expected. Parameters such as chlorophyll a and b content, the carotenoid/chlorophyll ratio, the photosynthetic compensation point and maximal rate of photosynthesis were not significantly different. Since all the plants used in this study carry the Argenteum mutation, references to wild type (wt) and mutant plants only relate to the pcd2 mutation.
Leaf disks: dose-response curve and growth.
Leaf disks (n = 5–8; ∅ 6 or 7 mm; without the mid vein) were taken from young, still folded pea leaves of about 40% full-size. For the leaf disk growth experiment, disks were floated on 10 mM KCl (± 50 µM c,t-ABA (Fluka, Switzerland)) in a petri dish that was put on an orbital shaker at 50 rpm for 22.5 hr at 21°C. The disks were exposed to white light (fluence rate of 150 µmol m−2 s−1) or kept in the dark. For the dose-response curves, leaf disks were put in light and given 0, 5, 10, 20, 25, 50 or 100 µM ABA. Before and after the experiment, the diameter of the disks was measured with a digimatic caliper (Mitutoyo, CD-15CP, United Kingdom). In the growth experiment, biologically inactive t,t-ABA (Sigma, United States) was used as a control treatment. For statistical analyses a one-way ANOVA was used with p < 0.05 with Newmans-Keuls Multiple Comparison Test as post test in GraphPadPrism 4 for Macintosh; the number of replicates was three or four. The dose-response curve was repeated three times and was fitted by a one-phase exponential decay function performed in GraphPadPrism 4 for Macintosh.
Leaf epidermal pavement cell area expansion.
Under the same conditions as in the leaf disk area growth experiment, cell growth of abaxial pavement cells was examined in four replicates. A section of one half of a young, unfolded leaf was placed on the experimental solution. From the other half of the same leaf the abaxial epidermal layer was gently removed and put on a microscopic slide with a drop of 10 mM KCl solution and sealed with medical adhesive B liquid (Aromado Medizintechnik GmBH, Düsseldorf, Germany). Digital images were taken (Coolpix 900, Nikon) of five or six sections per treatment. SigmaScanPro 5.0 (SPSS Science, Chicago, United States) was used to measure cell areas (ten cells per image). After 22.5 hr the epidermal layer of the leaf sections of the first half of the leaf was also gently removed and analyzed similar to the ones of the control treatment. For statistical analysis, see leaf disk growth.
Apoplastic acidification of epidermal strips measured with flat-tipped pH electrode.
Apoplastic acidification was measured in vivo with a flat-tipped pH electrode on abaxial epidermal strips. The strips were peeled from the leaf surface and mounted between two plexiglas plates (5 × 7 cm). In one of these plates a hole with a diameter of 7 mm was drilled providing access to the mesophyll side of the epidermal strip. The hole was filled with 1 ml of 1 mM KCl solution. The starting solution was changed by a flow-through system by using 10 ml of the new solution to assure a complete replacement. A flat-tipped combination pH-reference electrode (Thermo Orion 9167 SC, Beverly, MA, United States) was gently placed in contact with the mesophyll side of the epidermal strip through the hole. The pH was continuously recorded on a chart recorder. Blue light was provided by a projector lamp, filtered for blue light with a DT Blau filter (central wavelength 420 nm, Balzers, Maarssen, The Netherlands) and a calflex filter to lower infrared radiation. The fluence rate was measured with a light quantum meter (Quantitherm QRT 1, Hansatech Instruments Ltd., King's Lynn, United Kingdom) at the level of the epidermal strip.
Preceding the first light treatment, the epidermal strip was incubated for at least 1 hr in darkness. The solution was essentially unbuffered, resulting in differences in initial pH (6.56 ± 0.34, n = 9). During incubation in darkness, a steady, but very slow, acidification was observed. With intervals of 30 min, light pulses with a duration of 30 s and an intensity of 100 µmol m−2 s−1 blue light were given (Fig. 1A). The acidification rate is given by the difference between the rate over 10 min in the dark before the light pulse and the initial rate after exposure to light (Fig. 1B, line a and b, respectively). The effect of 20 µM ABA was determined by comparing the average acidification rate induced by three blue light pulses in control solution, with the average of three blue light pulses given in the presence of ABA, on the same epidermal strip. The reversibility of the inhibition of the acidification by ABA was determined by replacing the ABA-containing solution by the control solution again, and determining the average of three blue light-induced acidification rates. For statistical analyses the student-t-test was used, one-tailed on 95% confidence interval between the different treatments; the number of replicates in these experiments was five or six.
Although epidermal pavement cells of Argenteum leaves do contain some chloroplasts, Staal et al.15 showed that the inhibition of photosynthesis by DCMU, an inhibitor of photosystem II, does not affect the light-induced apoplastic acidification. Elzenga et al.18 showed that the pH changes observed were due to the pavement cells and not due to the guard cells.
Apoplastic acidification by epidermal strips with microelectrode ion flux estimations (MIFE); measurement of proton flux.
The MIFE™ technique,24,25 using proton-selective vibrating microelectrodes, was used to measure the net proton flux non-invasively. Microelectrodes were pulled from borosilicate glass (GC150-10, Harvard Apparatus LTD, Edenbridge, United Kingdom). The electrode blanks were silanized with tributylchlorosilane (FLUKA 90974, Zwijndrecht, The Netherlands), backfilled with a solution of 15 mM NaCl and 40 mM KH2PO4, and front-filled with Hydrogen Ionophore II, Cocktail A (FLUKA 95297, Zwijndrecht, The Netherlands). After a three-point calibration, electrodes with a response between 50 and 59 mV per pH unit and with a correlation coefficient between 0.999 and 1.000 (pH range 5.1–7.8) were used.
An epidermal strip, with the mesophyll side facing the 1 mM KCl experimental solution, was mounted around a glass capillary with medical adhesive. The capillary was fixed on the bottom of the experimental chamber with some grease at both ends. The chamber was placed on an inverted microscope (Nikon TMS-F, Uvikon, Bunnik, The Netherlands).
The proton-selective microelectrode, fixed in an electrode holder (MEH6SF15, WPI, Berlin, Germany), was mounted in a holder (MMT-5, Narishige, Tokyo, Japan) on a three-way piezo-controlled micromanipulator (PCT, Luigs & Neumann, Ratingen, Germany) that was driven by a computer-controlled stepper motor (MO61-CE08, Superior Electric, Bristol, CT, United States). During measurements the position of the electrode was alternating between 10–50 µm (both in the unstirred layer) from the epidermal surface with a frequency of 0.1 Hz. The chemical activity of protons in solution was continuously recorded at the two positions. Assuming a planar geometry and only diffusion of the ions in the unstirred layer, the proton fluxes and pH were calculated by subtracting the proton concentration determined at position x1 and x2. The pH values are the mean of the pH at the two positions at which the electrode measures. At intervals of 30 min, pulses of 30 s 100 µmol m−2s−1 blue light were applied. The effect of 20 µM ABA on the blue light-induced apoplastic acidification (Fig. 1B, line a and b) was determined as described before. For determining the light-induced effect on the proton flux, a basal line was drawn in the graph by eye (Fig. 1C, line c) and the maximal flux change was determined (double-headed arrow). The recovery of the initial acidification rate of the blue light response was determined by replacing the experimental solution with ABA by experimental solution without ABA and applying two or three blue light pulses afterwards. The ABA effect or recovery was expressed as percentage treatment of first blue light-induced control treatments before ABA was added. An average of three (wt + ABA), four (wt + t,t ABA) or nine (pcd2 + ABA) experiments was performed. For statistical analyses see leaf disk growth.
Results
Effect of ABA on light-induced leaf growth and leaf cell growth.
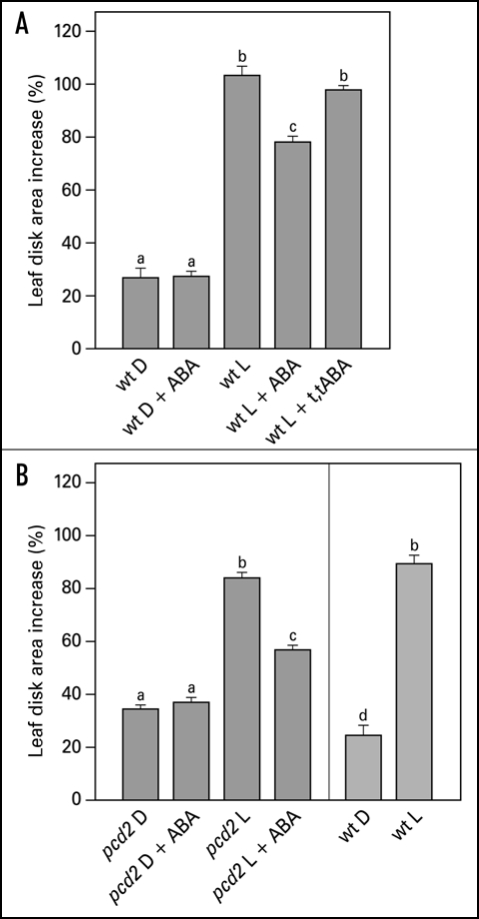
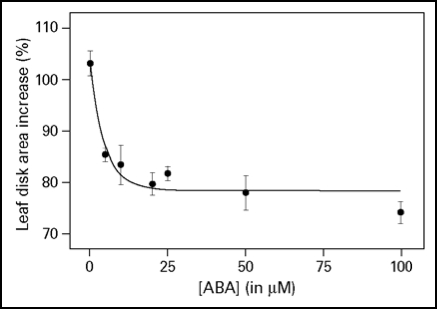
Integration of different environmental cues is important for plants to survive. The light-induced leaf expansion and the ABA-induced leaf area reduction can be taken as an example of balancing contradictory constraints in plant development. In light, leaf disks more than double in size and can be inhibited by the biologically active c,t-abscisic acid (ABA) (Fig. 3A). ABA inhibits white light-induced growth in a dose-responsive manner up to 25 µM ABA (Fig. 2). The dose-response relation of the inhibition by ABA is characterized by a Ki of 3.3 µM and maximal inhibition is found above 25 µM ABA. The biologically inactive ABA isoform t,t-ABA, is not inhibitory. In darkness, leaf disks grow about 20% and ABA has no inhibitory effect (Fig. 3A). ABA also inhibits the light-induced response of the Phytochrome Chromophore Deficient mutant pcd2 (Fig. 3B) to the same extent as in wild type. ABA also inhibits the light-induced expansion of epidermal pavement cells by 23% (Fig. 4).
Figure 3.
Effect of ABA on light-induced leaf disk growth of young expanding pea leaves of wt (A) and pcd2 (B). Leaf disks were floating on 10 mM KCl in darkness or in white light for 22.5 hr in the presence or absence of 50 µM ABA or the biologically inactive isoform t,t-ABA (50 µM). Growth is expressed as explained in the legend of Figure 2. The values are means ± SE of at least four independent experiments. The letters above the bars indicate the statistical group of the results from a one-way ANOVA, with Newman-Keuls as post test, α = 0.05. D = dark; L = light (150 µmol m−2s−1).
Figure 2.
Dose-response curve of ABA on light-induced leaf disk growth. Leaf disks were floating on 10 mM KCl in white light for 22.5 hr and given ABA in the concentration range of 0–100 µM. The growth is expressed as the percentage area increase during the 22.5 h of treatment compared with the size of the disk at the start of the experiment. The averaged data ± SE of three replicates are given, which fitted a one-phase exponential decay function Y = 24.5 * 10(−0.208X) + 78.5 with an r2 = 0.834.
Figure 4.
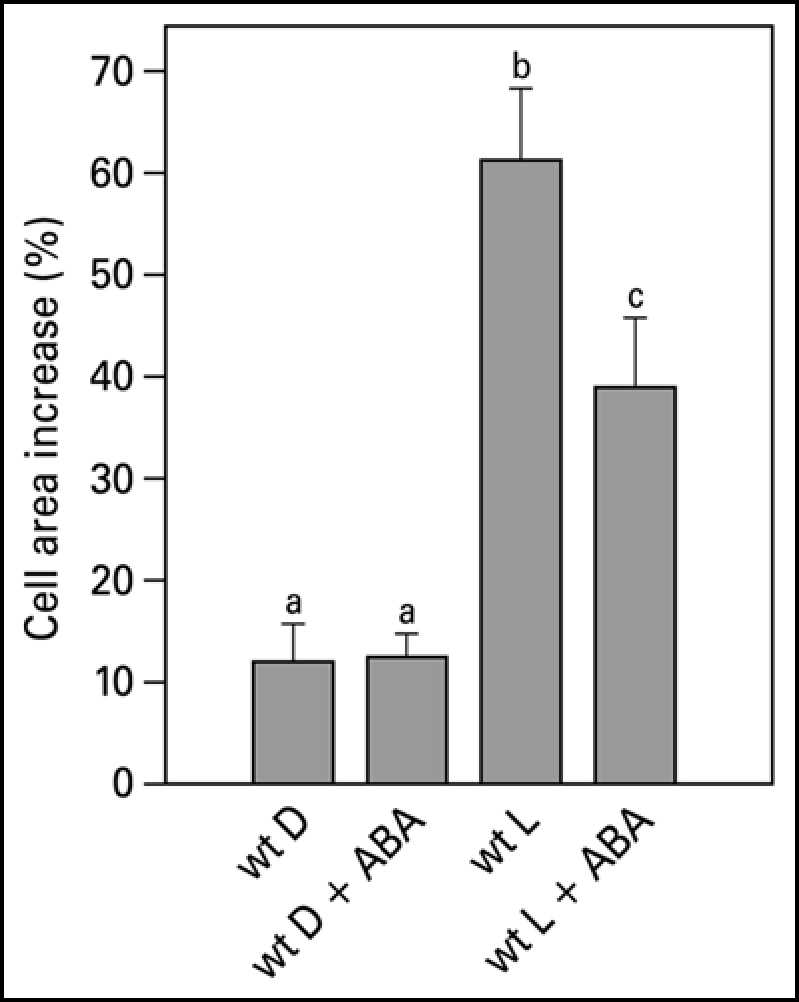
Pavement cell growth of the abaxial side of young expanding pea leaves of wt. Leaf strips were floating on a 10 mM KCl solution in darkness or in white light for 22.5 hr in the presence or absence of 50 µM ABA. Growth is expressed as explained in the legend of Figure 2. The values are means ± SE of at least four independent experiments. The letters above the bars indicate the statistical group of the results from a one-way ANOVA, with Newman-Keuls as post test, α = 0.05. D = dark; L = light (150 µmol m−2s−1).
Effect of ABA on blue light-induced apoplastic acidification.
For cell expansion to occur, generally, cell wall extensibility has to increase, while turgor is maintained. In the acid growth theory excretion of protons plays a central role in both of these processes. Measuring apoplastic acidification can show us whether ABA is inhibitory in leaf expansion by regulating proton excretion. Both red light and blue light induce apoplastic acidification,6,15 however, they have different response kinetics. A blue light pulse induces a fast response within two minutes and the pH is back to basal levels within half an hour, after which a second pulse will induce an identical response (Fig. 1). We made use of this fast and repetitive BL response to study the effect of ABA on the cell wall acidification. With the conventional pH electrode, application of 20 µM ABA resulted in an inhibition of the blue light-induced acidification (Fig. 5). This inhibitory effect can also be found with the MIFE technique, although the extent of the inhibition is slightly different. The biologically inactive t,t-ABA isoform did not have an effect on apoplastic acidification (Fig. 6A). In pcd2 ABA also inhibited the blue light-induced apoplastic acidification (Fig. 6A) and decreased the proton fluxes (Fig. 6C). When ABA is removed the blue light-induced apoplastic acidification (Fig. 6B) and proton fluxes (data not shown) recovered within an hour in all the apoplastic acidification experiments.
Figure 5.
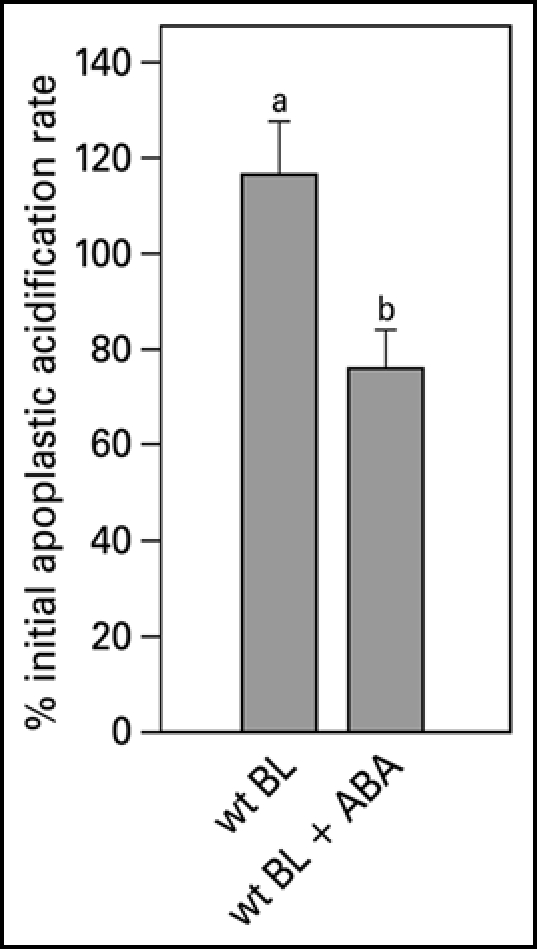
Blue light-induced relative apoplastic acidification rate of the abaxial epidermal layer of young expanding wt pea leaves measured with a conventional flat-tip pH-electrode in the presence or absence of 20 µM ABA. The apoplastic acidification rate is expressed as the percentage of the average of the acidification rate as induced by three blue light pulses given at the start of the experiment in control solution. Values are the means ± SE of at least five independent experiments. The letters above the bars indicate the statistical group of the results from a one-way ANOVA, with Newman-Keuls as post test, α = 0.05. BL = blue light (100 µmol m−2s−1).
Figure 6.
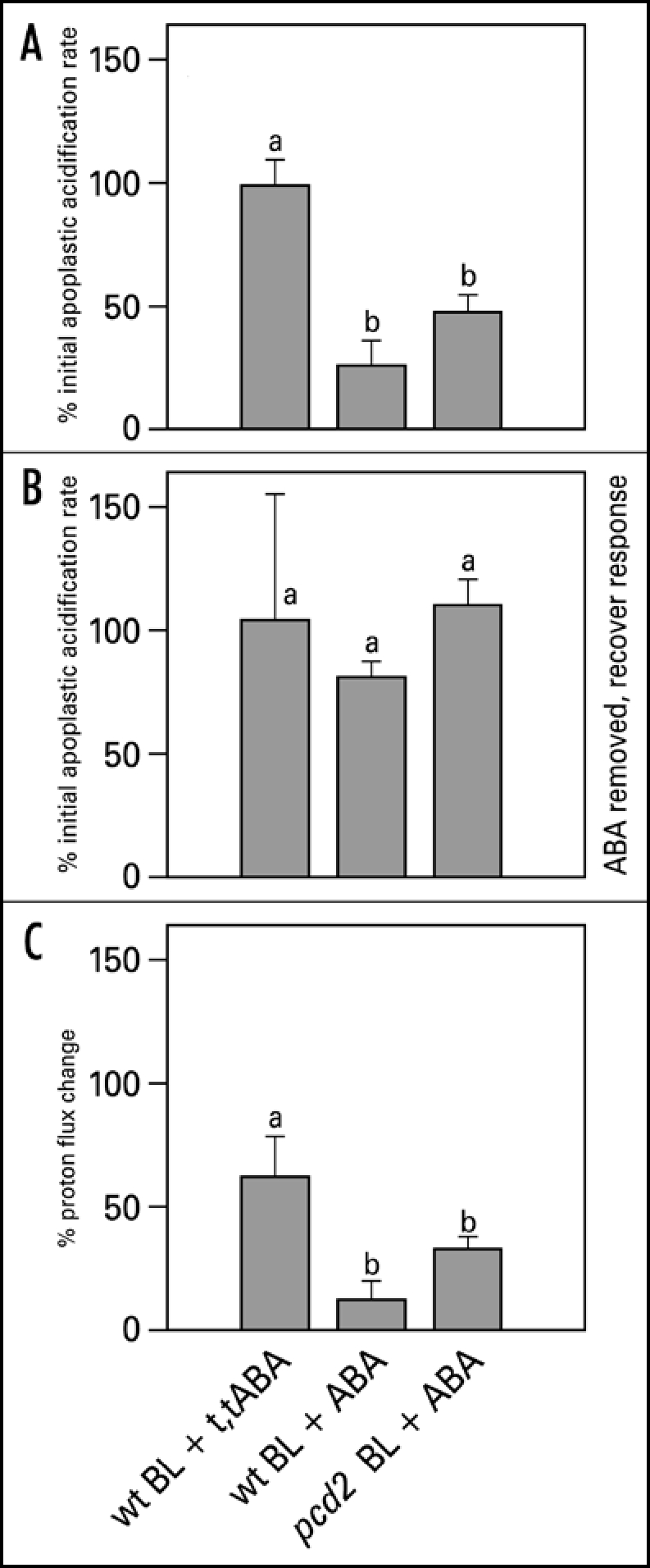
Blue light-induced relative apoplastic acidification rate and proton fluxes of abaxial epidermal strips of young expanding leaves of wt and pcd2 in the presence or absence of 20 µM ABA or t,t-ABA, measured with MIFE. (A) The effect of ABA and t,t-ABA on initial acidification rate in wt and pcd2. (B) Recovery of the blue light-induced apoplastic acidification rate after ABA was removed from the experimental solution. (C) The effect of the ABA and t,t-ABA on blue light-induced proton fluxes in wt and pcd2 mutants. The data are presented as the percentage of the average of the response induced by three blue light pulses given at the start of the experiment in control solution. Values are the means ± SE of at least three independent experiments. The letters above the bars indicate the statistical group of the results from a one-way ANOVA, with Newman-Keuls as post test, α = 0.05. BL = blue light (100 µmol m−2s−1).
Discussion
Effect of ABA on white light-induced leaf and epidermal pavement cell expansion.
Abscisic acid (ABA) induces water preserving reactions such as stomatal closure26 and the reduction of leaf expansion,21 with the latter process most sensitive to a lowered water potential.20 Whereas the mechanism of ABA regulation of stomatal closure is known in detail,27–29 it is unknown how ABA interferes with light-induced leaf growth. This study shows that ABA interacts with the light-induced leaf expansion by interfering with the signal transduction pathway from light receptor to proton pumping ATPase.
Light-induced leaf disk growth is inhibited by ABA (Fig. 3A). The growth response is not completely inhibited, but dose-response curves show that 25 µM ABA induces a maximal growth inhibition of 22% (Fig. 2). Based on the dose-response curve, a concentration of 50 µM, guaranteeing a maximal inhibitory effect, was used in the subsequent growth experiments. The physico-chemical characteristics of ABA (a weak acid) could be ruled out to play a role in leaf growth inhibition as t,t-ABA, a biological inactive isomer, had no effect on the growth response.
Leaf area increase is strongly correlated to a rapid cell area increase of the pavement cells.30 The reduction in leaf expansion of a phytochrome B mutant of pea under red light could be explained fully by a reduction in pavement cell area.8 Accordingly, it was expected that the effect of light and ABA on leaf disk expansion would be reflected in an equal effect on pavement cell expansion. Qualitatively we find that treatments that stimulate leaf growth also stimulate the expansion of epidermal cells. However, the effect of ABA on light-induced leaf disk growth is different from the effect of ABA on the light-induced expansion the pavement cells of isolated epidermal strips (Table 1). Based on careful examination and measurements of the individual cell sizes, we conclude that this discrepancy is due to a reduction of the size of pavement cells in the x and y direction of about 8% (±3, n = 101). No effect was found on the size of the stomatal complex (data not shown). Apparently, even gentle peeling causes the pavement cells to deform, as the turgor will induce the cells to take on a more rounded form, resulting in a smaller cell area, when not attached to the underlying mesophyll cell. When taking this into account, the effects of light and ABA on pavement cells and leaf disks are similar.
Table 1.
The comparison of leaf disk and pavement cell growth after 22.5 hrs white light ± 50 µM ABA treatment
| Treatment | Leaf Area Growth | Cell Area Growth | Difference in Area |
| Light | 103% | 61% | 21% |
| Light + ABA | 78% | 39% | 22% |
MIFE and pH electrode.
Both the flat-tipped pH electrode and the MIFE technique measure apoplastic pH. All the processes that influence the pH will affect measurements made by the stationary flat-tipped electrode. One of these processes is the vectorial transport of protons across the plasma membrane mediated by the proton-pumping ATPase. Another process is the so-called strong ion effect.31 A third process could be the change in [CO2], through respiration or photosynthesis. One of the advantages of using the MIFE system is that it focuses on vectorial processes. When the pH change is measured, and it is accompanied by an equal change in proton flux (Fig. 1B and C), it is not likely that processes other than proton pumping are involved in the acidification. As in both the pH measurements (Fig. 6A) and the proton flux measurements (Fig. 6C) the inhibition by ABA shows the same trend, we can conclude that the light-induced acidification depends on the vectorial transport of protons and that the effect of ABA solely depends on the modification of this ATPase-mediated, vectorial transport.
The inhibitory effect of ABA on the blue light-induced apoplastic acidification rate is stronger when determined by the MIFE technique (Figs. 5 and 6A) than with the flat-tipped pH electrode. This difference might be due to the exposure of the epidermal layer in the MIFE measuring chamber in which the tissue is more exposed to the medium than in the pH-electrode set up where pH electrode is placed gently against the epidermal layer. Although the experimental solution is changed in both experimental set-ups by flushing the system with several volumes of the measuring chamber, it is possible that the tissue is more exposed to ABA in the MIFE configuration.
Apoplastic acidification as short-term blue light response in leaf expansion.
In contrast to the 24 hr leaf growth experiments, the apoplastic acidification responses are short-term, virtually ruling out the more long-term effects of changes in gene expression and translation. The initial blue light-induced acidification rate in both wt (Figs. 5 and 6A) and in pcd2 (Fig. 6A) is inhibited by ABA. Since, (a) the light-induced acidification depends on proton pumping ATPase activity,15 (b) the measurements with MIFE indicate that ABA affects the vectorial proton transport and, (c) as t,t-ABA is ineffective, we can rule out any buffering or acidification effect of ABA itself, it can be concluded that ABA affects light-induced leaf growth via modulation of the ATPase activity. As the inhibition of apoplastic acidification by ABA is effective within minutes and proved fully reversible (Fig. 6B), this could be the mechanism by which the leaf growth rate is adjusted to short-term environmental changes.
ABA effects in the pcd2 mutant.
ABA inhibits the light-induced leaf growth in the pcd2 mutant of pea, in which the phytochrome-dependent signal transduction is almost completely switched off,8,32 to the same extent as it does in wt plants (Fig. 3). As there is no difference between wt and pcd2 in their response to ABA for growth, light-induced acidification rate, or light-induced proton fluxes it appears that ABA does not interact with the PCD2-dependent signaling pathway during blue light stimulation. Experiments with additional low red light illumination and/or blue light receptor mutants are needed to rule out the possibility that ABA may interact with the phytochrome-signaling side of the pathway.
Interaction of blue light-activated pathways and ABA.
In a dynamic model blue light was assumed to activate separate, but interacting signal transduction pathways, whereby phytochrome was assumed to be a component of the HF response.16 ABA can theoretically modify the signal transduction pathways at three different sites (Fig. 7): (1) in LF response pathway, involving phytochrome and which is dependent on calmodulin18 and supposedly on cytoplasmic calcium, which is a known second messenger in signal transduction pathways, (2) at the HF or blue light receptor pathway or (3) somewhere after the point where the two pathways are converged, including the proton-pumping ATPase itself. It is not expected that ABA affects the blue light-induced ATPase activity itself in a direct way. In Vicia faba guard cell protoplasts ABA also inhibits the blue light-induced proton pumping and causes a membrane depolarization, but when examined in microsomal vesicles, ABA does not affect the proton pumping.33 In Arabidopsis suspension cells, ABA also induces a membrane depolarization as a result of decreased proton pump activity34 dependent on [Ca2+]cyt. In our study the effect of ABA is most likely independent of [Ca2+]cyt, because ABA still inhibits the blue light-induced apoplastic acidification in pcd2. The results clearly show an interaction between ABA and the low fluence blue light-dependent siginaling pathway, while it still has to be demonstrated whether or not ABA affects the high fluence phytochrome-dependent pathway.
Figure 7.
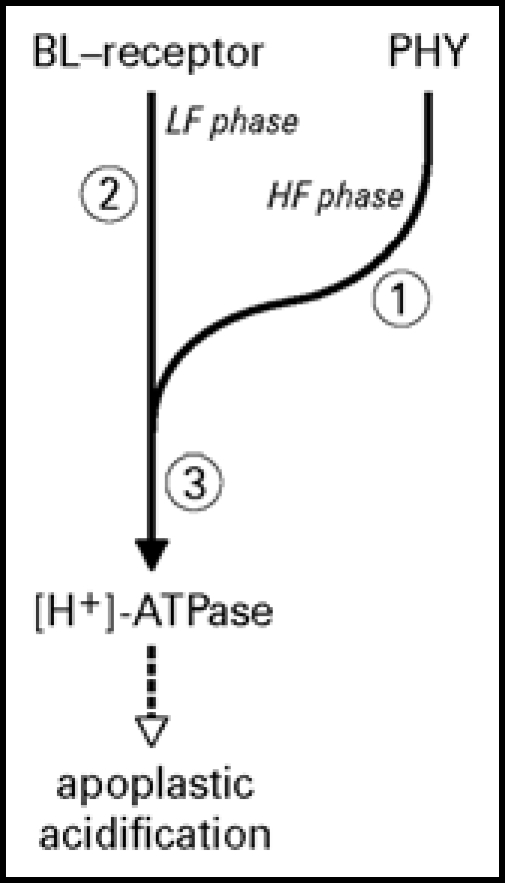
Model of the blue light signal transduction pathway of apoplastic acidification and the possible sites of ABA interaction. This model is based on the biphasic dynamic model, characterized by a high fluence (HF) component dependent on phytochrome and calmodulin, and a low fluence component (LF) independent of phytochrome and calmodulin.16 ABA can interact with the pathway at three potential sites: (1) at the phytochrome component which is dependent on calmodulin,18 (2) at the blue-light receptor-mediated pathway or (3) somewhere after the step where the two pathways are converged towards proton pump activation. By using the phytochrome deficient mutant pcd2 we have demonstrated that ABA does interact with the signaling pathway at 2 or 3. Whether ABA also affects the phytochrome-dependent pathway still remains to be determined.
Acknowledgements
We thank Johanneke Oosten for preliminary studies with the MIFE technique. This research was supported financially by a CEES stimulation grant.
Abbreviations
- ABA
c,t-ABA
- t,t ABA
t,t-ABA
- BL
blue light
- [Ca2+]cyt
cytoplasmic calcium
- MIFE
microelectrode ion flux estimation
- wt
Argenteum mutant of pea
- pcd2
phytochrome chromophore deficient mutant crossed with Argenteum mutant of pea
- SE
standard error
- HF
high fluence
- LF
low fluence
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=4314
References
- 1.Nagy F, Schafer E. Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants. Ann Rev Plant Biol. 2002;53:329–355. doi: 10.1146/annurev.arplant.53.100301.135302. [DOI] [PubMed] [Google Scholar]
- 2.Lin CT, Shalitin D. Cryptochrome structure and signal transduction. Ann Rev Plant Biol. 2003;54:469–496. doi: 10.1146/annurev.arplant.54.110901.160901. [DOI] [PubMed] [Google Scholar]
- 3.Spalding EP, Folta KM. Illuminating topics in plant photobiology. Plant Cell Environ. 2005;28:39–53. [Google Scholar]
- 4.Moller SG, Ingles PJ, Whitelam GC. The cell biology of phytochrome signaling. New Phytol. 2002;154:553–590. doi: 10.1046/j.1469-8137.2002.00419.x. [DOI] [PubMed] [Google Scholar]
- 5.Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8:217–230. doi: 10.1038/nrg2049. [DOI] [PubMed] [Google Scholar]
- 6.Blum DE, Elzenga JTM, Linnemeyer PA, Van Volkenburgh E. Stimulation on growth and ion uptake in bean leaves by red and blue light. Plant Physiol. 1992;100:1968–1975. doi: 10.1104/pp.100.4.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale JE. The control of leaf expansion. Ann Rev Plant Physiol Plant Mol Biol. 1988;39:267–295. [Google Scholar]
- 8.Elzenga JTM, Staal M, Prins HBA. Red light-induced acidification by pea leaf epidermal cells is regulated by more than one phytochrome. Phyton-Annales Rei Botanicae. 2000;40:35–44. [Google Scholar]
- 9.Savaldi-Goldstein S, Peto C, Chory J. The epidermis both drives and restricts plant shoot growth. Nature. 2007;446:199–202. doi: 10.1038/nature05618. [DOI] [PubMed] [Google Scholar]
- 10.Van Volkenburgh E. Leaf expansion -an integrating plant behaviour. Plant Cell Environ. 1999;22:1463–1473. [Google Scholar]
- 11.Van Volkenburgh E, Cleland RE. Proton excretion and cell expansion in bean leaves. Planta. 1980;148:273–278. doi: 10.1007/BF00380038. [DOI] [PubMed] [Google Scholar]
- 12.Rayle DL, Cleland RE. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Volkenburgh E, Cleland RE. Light-stimulated cell expansion in bean (Phaseolus vulgaris L.) leaves. I. Growth can occur without photosynthesis. Planta. 1990;182:72–76. doi: 10.1007/BF00239986. [DOI] [PubMed] [Google Scholar]
- 14.Van Volkenburgh E, Cleland RE, Watanabe M. Light-stimulated cell expansion in bean (Phaseolus vulgaris L.) leaves. II. Quantity and quality of light required. Planta. 1990;182:77–80. doi: 10.1007/BF00239987. [DOI] [PubMed] [Google Scholar]
- 15.Staal M, Elzenga JTM, Van Elk AG, Prins HBA, Van Volkenburgh E. Red and blue light-stimulated proton efflux by epidermal leaf cells of the Argenteum mutant of Pisum sativum. J Exp Bot. 1994;45:1213–1218. [Google Scholar]
- 16.Elzenga JTM, Staal M, Prins HBA. Modulation by phytochrome of the blue light-induced extracellular acidification by leaf epidermal cells of pea (Pisum sativum L.): A kinetic analysis. Plant J. 2000;22:377–389. doi: 10.1046/j.1365-313x.2000.00748.x. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad M, Jarillo JA, Smirnova O, Cashmore AR. The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Molecular Cell. 1998;1:939–948. doi: 10.1016/s1097-2765(00)80094-5. [DOI] [PubMed] [Google Scholar]
- 18.Elzenga JTM, Staal M, Prins HBA. Calcium-calmodulin signaling is involved in light-induced acidification by epidermal leaf cells of pea, Pisum sativum L. J Exp Bot. 1997;48:2055–2060. [Google Scholar]
- 19.Boyer JS. Leaf enlargement and metabolic rates in corn, soybean, and sunflower at various leaf water potentials. Plant Physiol. 1970;46:233–235. doi: 10.1104/pp.46.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsiao TC, Acevedo E. Plant responses to water deficits, water-use efficiency, and drought resistance. Agric Meteorol. 1974;14:59–84. [Google Scholar]
- 21.Van Volkenburgh E, Davies WJ. Inhibition of light-stimulated leaf expansion by abscisic acid. J Exp Bot. 1983;34:835–845. [Google Scholar]
- 22.Zhang J, Davies WJ. Changes in the concentration of ABA in xylem sap as a function of changing soil-water status can account for changes in leaf conductance and growth. Plant Cell Environ. 1990;13:277–285. [Google Scholar]
- 23.Hoch HC, Pratt C, Marx GA. Sub-epidermal air spaces — Basis for the phenotypic expression of the Argenteum mutant of Pisum. Am J Bot. 1980;67:905–911. [Google Scholar]
- 24.Newman IA. Ion transport in roots: Measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ. 2001;24:1–14. doi: 10.1046/j.1365-3040.2001.00661.x. [DOI] [PubMed] [Google Scholar]
- 25.Shabala SN, Newman IA, Morris J. Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol. 1997;113:111–118. doi: 10.1104/pp.113.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kriedema PE, Fuller GL, Loveys BR, Leopold AC. Abscisic acid and stomatal regulation. Plant Physiol. 1972;49:842. doi: 10.1104/pp.49.5.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Wang H, Takemiya A, Song CP, Kinoshita T, Shimazaki K. Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol. 2004;136:4150–4158. doi: 10.1104/pp.104.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- 29.MacRobbie EAC. Signaling in guard cells and regulation of ion channel activity. J Exp Bot. 1997;48:515–528. doi: 10.1093/jxb/48.Special_Issue.515. [DOI] [PubMed] [Google Scholar]
- 30.Maksymowych R. Abercrombie M, Newth DR, Torrey J. Analysis of leaf development. Cambridge: Cambridge University Press; 1973. Cell enlargement and differentiation; pp. 50–58. [Google Scholar]
- 31.Stewart PA. How to understand acid-base: A quantitative acid-base primer for biology and medicine. New York: Elsevier; 1981. [Google Scholar]
- 32.Weller JL, Terry MJ, Reid JB, Kendrick RE. The phytochrome-deficient pcd2 mutant of pea is unable to convert biliverdin IX alpha to 3(Z)-phytochromobilin. Plant J. 1997;11:1177–1186. doi: 10.1105/tpc.8.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goh CH, Kinoshita T, Oku T, Shimazaki KI. Inhibition of blue light-dependent H+ pumping by abscisic acid in Vicia guard-cell protoplasts. Plant Physiol. 1996;111:433–440. doi: 10.1104/pp.111.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brault M, Amiar Z, Pennarun AM, Monestiez M, Zhang ZS, Cornel D, Dellis O, Knight H, Bouteau FO, Rona JP. Plasma membrane depolarization induced by abscisic acid in Arabidopsis suspension cells involves reduction of proton pumping in addition to anion channel activation, which are both Ca2+ dependent. Plant Physiol. 2004;135:231–243. doi: 10.1104/pp.104.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]