Abstract
The immune response of plants to potential pathogens consists of two levels of defense; basal resistance triggered by pattern recognition (PTI) and effector triggered immunity (ETI). Recent analysis of the Arabidopsis proteome after challenge by three strains of Pseudomonas syringae identified proteins implicated in the establishment of disease, PTI and ETI. In this addendum we discuss the significance of some of the putative post-translational modifications and the predicted localisation of these proteins. We speculate on the apparent bias of chloroplast targeted proteins amongst those identified and consider the strengths and weaknesses inherent to a comparative proteomics approach.
Key Words: proteomics, Arabidopsis, defense, pathogen, post-translational modifications, Pseudomonas
Manipulation of a host's metabolism by microbial pathogens to enable their survival is a finely balanced interaction. Plant and animal cells can recognise conserved features of microbes, such as bacterially derived flagellin, lipopolysaccharides or fungal chitin using receptors, for example, the flagellin-sensing receptor, FLS2. In plants, these recognition events trigger basal defense responses such as cell wall thickening and acidification of the apoplast that restrict the growth of the pathogen.1 Upon contact with a host cell, gram negative phytopathogenic bacteria, such as Pseudomonas syringae, introduce a suite of proteins into host cells through a type III secretion system.2 These type III effector proteins (T3E) have been shown to suppress basal defenses and enhance conditions for pathogen growth and reproduction, e.g., AvrRpm1 and AvrRpt2,3 AvrPto4 and AvrPtoB.5 However, T3E proteins or their actions, may betray the pathogen to a new level of host defenses. Specialised host proteins can either directly recognise effector proteins (for example, Pto and AvrPto),6 or may detect perturbation of defense-related proteins, (the guard hypothesis exemplified by RIN4),7 triggering a rapid and vigorous second layer of defense, known as resistance-gene mediated defense. A ‘zigzag’ model has been proposed to summarise and integrate these levels of plant immunity.8
In a recent study we reported modifications to the defense proteome of Arabidopsis thaliana in response to challenge by three strains of Pseudomonas syringae pv tomato DC3000.9 Two-dimensional gel electrophoresis (2DE) was used to determine protein changes characteristic of establishment of disease, basal resistance and R-gene mediated defense by comparing responses to DC3000, a non pathogenic DC3000 hrp mutant and DC3000 expressing avrRpm1 respectively. Proteins were extracted from three subcellular fractions; total soluble protein, chloroplast-enriched and mitochondria-enriched across four time points. General trends emerging from this broad study indicated that defense related enzymes (glutathione-S-transferases (GSTs), preoxiredoxins, superoxide dismutase) and metabolic enzymes were altered in basal defense responses. Many changes associated with basal defense were modulated, presumably by effector proteins, in the compatible interaction (leading to disease). Some protein folding, or protein binding components showed changes in response to T3E (a 14-3-3 and cyclophillins). Many redox related systems showed changes in response to AvrRpm1/RPM1 interaction, including well known defense related proteins such a GSTs.10
Of the 52 proteins identified by Jones et al.,9 ten were observed in two or more spots. These proteins were three GSTs and several metabolic enzymes; glyceraldehyde-3-phosphate dehydrogenase, phosphoribulokinase, malate dehydrogenase, carbonic anhydrase 2 (CA2) and rubisco activase. Other proteins represented by more than one spot were a component of the oxygen evolving complex (OEC33) and a translationally-controlled tumour protein (TCTP). We generally attributed spot shifts to post translational modifications. An exception is CA2 (At5g14740) which occurs in two splice forms and the predicted Mw (28.3 and 36.6 kDa) of these forms are similar to those observed (29.7, 31.9 kDa). Rubisco activase may also occur in two splice forms. A weakness of using spot shifts in 2DE to identify proteins subject to post-translational modifications (PTM) is that limitations of solubility and resolution make it unlikely that a gel will contain all forms of a protein, even if all the spots on the gel were to be identified. PTMs are one reason why an increase in the density of a spot on a gel cannot, without additional evidence, be correlated to an increase in protein activity. It is possible that the observed spot represents a breakdown product or an inactivated form of a protein. For example, a flood-gate mechanism has been proposed for peroxiredoxins where these enzymes are inactivated by overoxidation of critical cysteine residues in their active sites.11 While such a theory remains contentious, different forms of these proteins can be readily observed.10,12
A naïve expectation was that the suppression of basal defenses would be evident in both DC3000 and the DC3000(avrRpm1) treatments when compared to the hrp mutant, as DC3000(avrRpm1) differs from DC3000 only by the addition of one effector. One unexpected result of this study was how few proteins responded in a similar manner in both DC3000 and the DC3000(avrRpm1) inoculations; 22 spots were identified as T3E responsive but only three proteins (peroxiredoxin A, hydroxybillane synthase and rubisco activase) showed a similar response with DC3000(avrRpm1). One consequence of AvrRpm1 introduction to a host cell is phosphorylation and subsequent disappearance of RIN4, triggering RPM1 mediated signalling and ensuing resistance.7 The magnitude of response to the addition of AvrRpm1 to a host cell is likely to be the result of activated RPM1 signalling rather than through the direct actions of AvrRpm1.
A high proportion (68%) of the proteins classified as modulated by T3Es are predicted to be targeted to the chloroplast. Obviously this relies on the accuracy of prediction tools, fails to consider the dynamic nature of protein localisation and seeks to return just one ‘address’ for a protein. A single location for a protein may simply be inaccurate, as the majority of organelle proteins are encoded in the nucleus and must traverse the cytoplasm, and many proteins are dual targeted to both the mitochondria and chloroplasts.13,14 Furthermore, the organelles themselves may exert transcriptional control over the nucleus, through redox related signalling.15 A speculation regarding the over-representation of chloroplast targeted proteins amongst T3E responsive proteins is that T3Es may preferentially target chloroplast components, possibly due to homology between bacterial and plastid systems. Alternatively, these changes may simply underscore the importance of the chloroplast in redox mediated signalling and the observed changes to proteins could be initiated by the host cell.
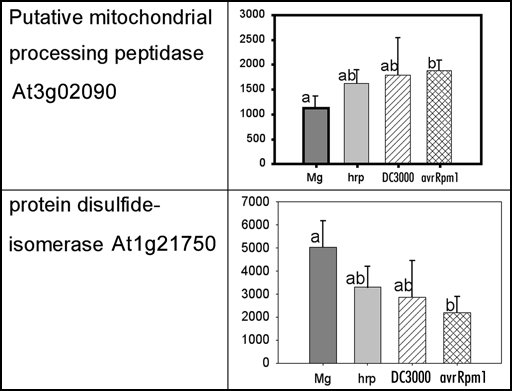
The dynamic nature of protein localisation and modification also limits proteomic studies where only static time points can be sampled. Ensuring that the material is as homogenous as possible may reduce variability, however, some potentially interesting proteins with established roles in defensive or signalling pathways may just fail statistical criteria for acceptance. For example, from the unpublished data of Jones et al, 2006, a processing peptidase (At3g02090) and a protein disulfide isomerase (At1g21750) show significant difference between the control (MgCl2) and the DC3000(avrRpm1) treatments, but the other two bacterial inoculations, DC3000 and DC3000 hrpA, are not significantly different from either (Fig. 1). Such changes are hard to interpret; are they just noise or do they really represent a dynamic change and the time point sampled just caught transient differences?
Figure 1.
Spot density of two proteins from the mitochondrial-enriched fraction at 3 hpi.
To conclude, we9 provided new insights into early post-transcriptional changes in the defense proteome, confirming that broad sweep proteomic analyses can reveal changes that are impossible to determine through transcriptomics alone. However such studies must consider the inherent limitations of 2DE. At best, such work reveals subjects for further targeted work such as silencing of selected genes, mutagenesis of PTM or localization motifs.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3684
References
- 1.Gomez-Gomez L, Felix G, Boller T. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J. 1999;18:277–284. doi: 10.1046/j.1365-313x.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- 2.Collmer A, Badel JL, Charkowski AO, Deng WL, Fouts DE, Ramos AR, Rehm AH, Anderson DM, Schneewind O, van Dijk K, Alfano JR. Pseudomonas syringae Hrp type III secretion system and effector proteins. Proc Natl Acad Sci USA. 2000;97:8770–8777. doi: 10.1073/pnas.97.16.8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim MG, da Cunha L, McFall AJ, Belkhadir Y, DebRoy S, Dangl JL, Mackey D. Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defense in Arabidopsis. Cell. 2005;121:749–759. doi: 10.1016/j.cell.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Hauck PR, Thilmony R, He SY. A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc Natl Acad Sci USA. 2003;100:8577–8582. doi: 10.1073/pnas.1431173100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Torres M, Mansfield JW, Grabov N, Brown IR, Ammouneh H, Tsiamis G, Forsyth A, Robatzek S, Grant M, Boch J. Pseudomonas syringae effector AvrPtoB suppresses basal defense in Arabidopsis. Plant J. 2000;47:368–382. doi: 10.1111/j.1365-313X.2006.02798.x. [DOI] [PubMed] [Google Scholar]
- 6.Cui JP, Jander G, Racki LR, Kim PD, Pierce NE, Ausubel FM. Signals involved in Arabidopsis resistance to Trichoplusiani caterpillars induced by virulent and avirulent strains of the phytopathogen Pseudomonas syringae. Plant Physiol. 2002;129:551–564. doi: 10.1104/pp.010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackey D, Holt BF, Wiig A, Dangl JL. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell. 2002;108:743–754. doi: 10.1016/s0092-8674(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 8.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 9.Jones AM, Thomas V, Bennett MH, Mansfield J, Grant M. Modifications to the Arabidopsis defense proteome occur prior to significant transcriptional change in response to inoculation with Pseudomonas syringae. Plant Physiol. 2006;142:1603–1620. doi: 10.1104/pp.106.086231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones AM, Thomas V, Truman B, Lilley K, Mansfield J, Grant M. Specific changes in the Arabidopsis proteome in response to bacterial challenge: Differentiating basal and R-gene mediated resistance. Phytochemistry. 2004;65:1805–1816. doi: 10.1016/j.phytochem.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 12.Woo HA, Won Kang S, Kim HK, Yang KS, Chae HZ, Rhee SG. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid: Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J Biol Chem. 2003;278:47361–47364. doi: 10.1074/jbc.C300428200. [DOI] [PubMed] [Google Scholar]
- 13.Chew O, Whelan J, Millar AH. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J Biol Chem. 2003;278:46869–46877. doi: 10.1074/jbc.M307525200. [DOI] [PubMed] [Google Scholar]
- 14.Millar AH, Whelan J, Small I. Recent surprises in protein targeting to mitochondria and plastids. Curr Opin Plant Biol. 2006;9:610–615. doi: 10.1016/j.pbi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Beck CF. Signaling pathways from the chloroplast to the nucleus. Planta. 2005;222:743–756. doi: 10.1007/s00425-005-0021-2. [DOI] [PubMed] [Google Scholar]