Abstract
Key requirements for microbes to initiate and establish mutualistic symbiotic interactions with plants are evasion of potential host defense responses and strict control of microbial growth. We have recently shown that reactive oxygen species (ROS) produced by a specific fungal NADPH oxidase isoform NoxA, have a critical role in regulating hyphal growth in the mutualistic interaction between Epichloë festucae and perennial ryegrass. Regulation of ROS production in the symbiosis requires two additional components, NoxR and RacA, homologues of the mammalian p67phox and Rac2. Perennial ryegrass host plants containing noxA or noxR mutants lose apical dominance, become severely stunted, and undergo precocious senescence. Our working model proposes that hyphal tip growth and branching is controlled by localized bursts of ROS catalysed by NoxA, following recruitment of NoxR and RacA from the cytosol to the membrane in response to signaling from the grass host.
Key Words: Epichloë festucae, Lolium perenne, NoxA, NADPH oxidase, reactive oxygen species, NoxR
Most, if not all, plants found in natural ecosystems form symbiotic associations with fungi that can range in space and time across a continuum from mutualism to antagonism. While much is known about the signals and mechanisms that lead to pathogenic interactions between fungi and plants, much less is known about how fungi maintain mutualistic interactions and what molecular events trigger the switch from mutualism to antagonism. Key requirements for initiation, establishment and maintenance of mutualistic interactions between fungi and plants are suppression or evasion of potential host defence responses and strict control of hyphal growth in the plant tissue. Symbiotic associations between endophytes of the Epichloë/Neotyphodium group and temperate grasses of the subfamily Pooideae, provide an ideal experimental system in which to study fungal-plant mutualism.1,2 These biotrophic fungi systemically colonise the intercellular spaces of aerial tissues of the vegetative and reproductive tillers, as well as the seed. For the most part these endophytes form mutualistic associations but during the reproductive phase of plant growth the sexual Epichloë spp. can form reproductive structures (stromata) around the developing inflorescence that partially or completely sterilize the host, by preventing emergence of the inflorescence. Host benefits from the symbiosis include improved growth, nutrient acquistion as well as enhancement of plant tolerance to biotic and abiotic stresses, such as drought, disease and animal herbivory. Endophyte benefits include access to nutrients from the plant apoplastic space and a mode of dissemination by vertical transmission through the seed.
Using the E. festucae-perennial ryegrass (Lolium perenne) symbiotum as our experimental system and a forward genetics approach, we recently identified a plasmid insertional mutant of E. festucae that was symbiotically defective.3 In wild-type associations, E. festucae grows systemically in the intercellular spaces as infrequently branched hyphae parallel to the axis of the leaf.4,5 Growth of the hyphae is synchronized with that of the host throughout the life cycle of the grass. Inactivation of a gene encoding a specific NADPH oxidase isoform, NoxA, resulted in unregulated growth of the fungal hyphae in meristematic and mature leaf tissue giving rise to a dramatic increase in fungal biomass in all tissues.3 Hyphae in the noxA mutant symbiotum showed an increase in vacuolation compared to wild-type. Plants infected with the noxA mutant lose apical dominance, become severely stunted, and show precocious senescence. While no evidence was obtained that the noxA mutant elicits a hypersensitive response (HR), further biochemical analysis of the host plant response is required to confirm that the plants infected with the noxA mutant undergo senescence rather than apoptosis. Expression analysis of plant genes known to be upregulated (SAG12) or downregulated (Cab) during senescence should help clarify the host biochemical phenotype.6 By contrast deletion of a second E. festucae NADPH oxidase gene, noxB, had no effect on the plant symbiotic interaction phenotype.3 Using transmission electron microscopy to locate deposits of electron-dense cerium perhydroxides we were able to show that H2O2 production was significantly reduced in the endophyte extracellular matrix and associated plant cell walls of meristematic tissue infected with the noxA mutant compared to wild-type. These results demonstrate that fungal production of ROS is critical for maintaining the mutualistic interaction between E. festucae and perennial ryegrass.
Production of a burst of superoxide catalysed by NADPH oxidase is an important defence mechanism in both mammals and plants.7,8 The most well characterized NADPH oxidase is the mammalian gp91phox (NOX2), which is responsible for high-level production of superoxide in phagocytic cells in response to microbial or inflammation signals. The discovery of four additional NADPH oxidases (NOX) and two dual oxidases (DUOX), and the demonstration that they are involved in a variety of cellular processes, including cell proliferation, apoptosis and hormone responses, has led to the recognition that ROS production is a ubiquitous signaling system to control various differentiation processes.9–11 The NADPH homologues in plants, designated Rboh (respiratory burst oxidase homolog), are structurally similar to the mammalian calcium regulated NOX5, which has an N-terminal calcium binding EF-hand motif. Arabidopsis thaliana possesses 10 NADPH oxidases, which have been shown by genetic analysis to be involved in a diverse range of plant cell processes, including host defence, and regulation of stomatal closure, seed germination and root hair development.12,13 In contrast, fungi have between one and three NOX isoforms.14 The fungal NoxA and NoxB are structurally similar to the mammalian gp91phox but appear to lack any additional fungus-specific domains. The single NOX identified in Aspergillus nidulans, NoxA, has been shown to be essential for fruiting body development.15 Deletion of the noxA homologues in Podospora anserina (nox1) and Neurospora crassa (nox-1), also disrupt sexual development.14,16 Disruption of the second nox found in P. anserina (nox2) and N. crassa (nox-2) impairs ascospore germination. This is consistent with a role for ROS in fungal cell differentiation.14 We have been unable to test whether the E. festucae noxA has a similar developmental role because the sexual cycle in this fungus can only be initiated on the plant during the reproductive phase of growth. The inability of symbiota containing the noxA mutant to survive precludes such an analysis. The two plant pathogenic fungi, Fusarium graminearum and Magnaporthe grisea, possess a third nox gene, noxC, which encodes a NOX with an N-terminal calcium binding motif, similar to that found in human NOX5 and the plant Rbohs.14 The absence of noxC homologues in the genomes of saprophytic fungi may indicate that this gene has a specific role in plant pathogenesis.
Generation of ROS by the phagocytic NOX requires formation of a multi-enzyme complex composed of the catalytic subunit gp91phox and the regulatory subunits p22phox, p40phox, p47phox, p67phox and the small GTPase Rac.17,18 A search of fungal genomes for homologues of these mammalian NOX components identified a gene encoding a protein with an N-terminal domain very similar to p67phox, with motifs for both RAC and gp91phox (NOX2) binding.19 This gene, which we have designated noxR, was cloned from E. festucae and a deletion mutant generated by targeted gene replacement. The plant interaction phenotype of symbiota containing the noxR mutant was very similar to that observed for the noxA mutant i.e., plants showed increased tillering, were stunted, and showed precocious senescence (Fig. 1).19 Hyphae in the meristematic tissues of noxR mutant associations were more highly branched, resulting in an increase in fungal biomass in these tissues, and in the leaf sheath and blade that arise from these tissues (Fig. 1). Overexpression of noxR, under the control of a TEF promoter, or depletion of ROS by growing wild-type cultures in the presence of DPI, disrupted normal apical tip growth and induced hyphal hyperbranching, a culture phenotype similar to that observed for the noxR mutant in planta. These results indicate that NoxR has a crucial regulatory role in controlling hyphal tip growth and branching in the grass host. However, NoxR alone is insufficient to activate NoxA in planta. By analogy with the mammalian phagocytic system, which requires both p67phox and Rac2 for gp91phox activation, E. festucae also requires a small GTP binding protein, RacA, for ROS production. Yeast two-hybrid and pull-down assays showed NoxR interacts with RacA. A single amino acid substitution in the predicted RacA binding site of NoxR (R101E), abolished the ability of NoxR to complement a noxR mutation in planta, indicating that both NoxR and RacA are required to activate NoxA in the host plant. Reconstitution of Nox activity either in vitro or in a mammalian cell culture system will demonstrate whether NoxR and RacA are necessary and sufficient to activate NoxA.
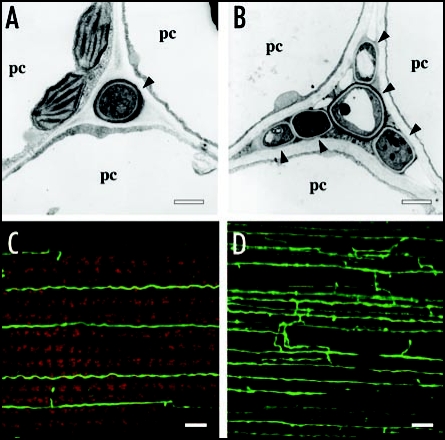
Figure 1.
In planta phenotype of E. festucae noxR mutant. (A and B) Transmission electron micrographs of cross sections of E. festucae Fl1 wild-type (A) and noxR mutant (B) hyphae (arrowheads) in the intercellular space of perennial ryegrass. pc: plant cell. Bars = 1 µm. (C and D) Confocal depth series images of hyphal morphology of GFP expressing E. festucae wild-type (C) and noxR mutant (D) in perennial ryegrass leaf blade Bars = 10 µm.
This work indicates that localized ROS production, catalysed by NoxA, is critical for controlling the highly regulated growth pattern observed for E. festucae in the meristematic and vegetative tissue of the perennial ryegrass host. The genetic evidence suggests that NoxR and RacA control the spatial and temporal activation of NoxA in planta, and by extension hyphal tip growth and branching. The NoxA mediated spatial patterning of hyphal tip growth and branching is analogous to ROS mediated regulation of root hair growth in plants. Roots of A. thaliana RbohC loss-of-function mutants (rhd2) have decreased levels of ROS at the root hair tip and reduced root hair growth.12 These mutants are also defective in Ca2+ uptake and therefore fail to establish a Ca2+ gradient at the root tip that is required for polarized growth. These results indicate that localized production of ROS, catalysed by the RbohC NADPH oxidase isoform, facilitates Ca2+ influx for growth, most likely through the activation of hyperpolarization-activated Ca2+ channels (HACC).20 The isolation of an A. thaliana RhoGDI mutant (scn1) that showed ectopic development of root hair initials on trichoblasts, established that Rho GTPases (called ROPs in plants), which are known to interact with RhoGDI, are a crucial component of the cell regulatory system for controlling spatial production of ROS.21 These results indicate that ROS has a key role in controlling plant cell growth, principally through cell expansion.
Although we have established a role for ROS in regulating hyphal growth in a fungal-grass mutualistic symbiosis many questions remain unanswered. Are there unique components of the Nox complex, yet to be identified, that are recruited from the cytosol to the membrane in response to plant signaling? The presence of protein-protein interaction domains in the C-terminus of NoxR that differ to those found in p67phox would suggest that additional fungal-specific components remain to be identified. What is the mechanism for activation and recruitment of RacA to the membrane, to activate NoxA? In animals RhoGDIs are known to be key regulators of Rho GTPase function, by regulating both the interactions of Rho GTPases with regulators and effector targets, as well as cytosol-to-membrane cycling.22 There is increasing evidence that kinases acting on either GDIs or Rho GTPases themselves, act to regulate formation of complexes. Lipids and phosphatases may also act in concert with kinases, to control the overall specificity and dynamics of Rho GTPase action.22 Further insights into how the fungal Rho GTPase-GDI cycle is regulated will help bridge the gap in our knowledge between epichloë endophyte sensing of plant signals and transduction of those signals within the cell to activate Nox. How ROS signal within the cell and in what molecular form, remains a key question to understand ROS mediated cellular differentiation processes. Two general mechanisms that link oxidants to several pathways are protein tyrosine phosphoryation cascades and intracellular Ca2+ transients.23 Tyrosine phosphorylation is widely used by multicellular organisms to control cell fate decisions and cytoskeleton dynamics.24 Both tyrosine kinases and protein tyrosine phosphatases (PTPs) exert signal specificity through subcellular targeting as well as catalytic domain specificity. The presence of an invariant cysteine residue in PTPs makes them particularly susceptible to oxidative inactivation. Both superoxide and peroxide are able to react with the catalytic cysteine residues of PTPs and cysteine rich regions of many transcription factors. Oxidants are also known to trigger the generation of Ca2+ signals, in part through activation of Ca2+ channels. Genetic evidence supports the hypothesis of a link between ROS production and Ca2+ signaling for at least two developmental systems; root hair development in Arabidopsis thaliana12,21 and multi-cellular development in Dictyostelium discoideum.10 Whether there is cross talk between ROS and Ca2+ signaling in filamentous fungal cell development remains to be determined.
In summary, we have shown that fungal NoxA, NoxR and RacA are all required to maintain a mutualistic symbiotic interaction between E. festucae and perennial ryegrass. Our working model proposes that hyphal tip growth and branching is controlled by localized bursts of ROS catalysed by NoxA, following recruitment of NoxR and RacA from the cytosol to the membrane in response to signaling from the grass host. It will be of considerable interest to determine how widespread this signaling mechanism has been adopted to control other fungal-plant symbiotic interactions.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3725
References
- 1.Schardl CL, Leuchtmann A, Spiering MJ. Symbioses of grasses with seedborne fungal endophytes. Annu Rev Plant Biol. 2004;55:315–340. doi: 10.1146/annurev.arplant.55.031903.141735. [DOI] [PubMed] [Google Scholar]
- 2.Scott B. Epichloë endophytes: Symbionts of grasses. Curr Opin Microbiol. 2001;4:393–398. doi: 10.1016/s1369-5274(00)00224-1. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka A, Christensen MJ, Takemoto D, Park P, Scott B. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic association. Plant Cell. 2006;18:1052–1066. doi: 10.1105/tpc.105.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen MJ, Bennett RJ, Schmid J. Growth of Epichloë/Neotyphodium and p-endophytes in leaves of Lolium and Festuca grasses. Mycol Res. 2002;106:93–106. [Google Scholar]
- 5.Tan YY, Spiering MJ, Scott V, Lane GA, Christensen MJ, Schmid J. In planta regulation of extension of an endophytic fungus and maintenance of high metabolic rates in its mycelium in the absence of apical extension. Appl Environ Microbiol. 2001;67:5377–5383. doi: 10.1128/AEM.67.12.5377-5383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gombert J, Etienne P, Ourry A, Le Dily F. The expression patterns of SAG12/Cab genes reveal the spatial and temporal progression of leaf senescence in Brassica napus L. with sensitivity to the environment. J Exp Bot. 2006;57:1949–1956. doi: 10.1093/jxb/erj142. [DOI] [PubMed] [Google Scholar]
- 7.Doke N. Involvement of superoxide anion generationin the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol. 1983;23:345–357. [Google Scholar]
- 8.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 9.Lambeth JD, Cheng G, Arnold RS, Edens WA. Novel homologs of gp91phox. Trends Biochem Sci. 2000;25:459–461. doi: 10.1016/s0968-0004(00)01658-3. [DOI] [PubMed] [Google Scholar]
- 10.Lardy B, Bof M, Aubry L, Paclet MH, Morel F, Satre M, Klein G. NADPH oxidase homologs are required for normal cell differentiation and morphogenesis in Dictyostelium discoideum. Biochim Biophys Acta. 2005;1744:199–212. doi: 10.1016/j.bbamcr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 12.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 13.Torres MA, Dangl JL, Jones JD. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguirre J, Rios-Momberg M, Hewitt D, Hansberg W. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 2005;13:111–118. doi: 10.1016/j.tim.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Lara-Ortiz T, Riveros-Rosas H, Aguirre J. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol Microbiol. 2003;50:1241–1255. doi: 10.1046/j.1365-2958.2003.03800.x. [DOI] [PubMed] [Google Scholar]
- 16.Malagnac F, Lalucque H, Lepère G, Silar P. Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet Biol. 2004;41:982–997. doi: 10.1016/j.fgb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nature Immunol. 2001;2:211–215. doi: 10.1038/85259. [DOI] [PubMed] [Google Scholar]
- 18.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 19.Takemoto D, Tanaka A, Scott B. A p67Phox-like regulator is recruited to control hyphal branching in a fungal-grass mutualistic symbiosis. Plant Cell. 2006;18:2807–2821. doi: 10.1105/tpc.106.046169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gapper C, Dolan L. Control of plant development by reactive oxygen species. Plant Physiol. 2006;141:341–345. doi: 10.1104/pp.106.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438:1013–1016. doi: 10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- 22.DerMardirossian C, Bokoch GM. GDIs: Central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Terada LS. Specificity in reactive oxidant signaling: Think globally, act locally. J Cell Biol. 2006;174:615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]