Abstract
Plants respond to local herbivory or pathogen infection with phenotypic changes, which reduce the danger of future attack. This so-called induced resistance is usually not restricted to the attacked plant organ but is also expressed in distant, so far undamaged parts of the plant. Signaling compounds such as jasmonic acid and salicylic acid have been discovered that move within the plant via the xylem or the phloem and elicit the resistance, thus acting as plant hormones. We now found that volatiles released in response to herbivore damage are required to elicit extrafloral nectar secretion in other parts of the same plant. Extrafloral nectar attracts ants and other carnivorous arthropods and serves as an effective indirect defense against herbivores. So called green leaf volatiles are released within minutes in response to tissue damage and were among the compounds that induced nectar secretion in yet undamaged parts of the damaged plant, but also in neighboring plants. Being gaseous and transported via the air, green leaf volatiles can serve a rapid within-plant communication, which moves much faster from one plant organ to the other than any plant-internal compound.
Key WordS: plant-insect interaction, extrafloral nectar, green leaf volatiles, Lima bean, induced resistance, induced defense, plant-plant communication, systemic resistance
Induced plant resistance traits are expressed in response to an eliciting attack. While pathogenesis-related proteins and phytoalexins are important means of induced systemic resistance to pathogens,1,2 induced resistance to herbivores comprises direct defenses and traits that improve the plant's interaction with the third trophic level.3 For instance, extrafloral nectar (EFN) is usually secreted on vegetative organs and attracts ants and other carnivorous arthropods, thereby increasing the predation pressure on herbivores.4 EFN secretion increases in response to mechanical damage or herbivore feeding5–9 and thus represents an induced indirect defense. Similarly, herbivore-induced volatiles (HI-VOCs) are released in response to herbivore damage and serve as defensive means by attracting carnivorous arthropods such as parasitoidic wasps.10–12 While the synthesis of PR proteins and phytoalexins depends on salicylic acid moving within the phloem and eliciting the systemic resistance,13 jasmonic acid (JA) has been identified as a hormone that is both sufficient and required to elicit induced defenses to herbivores.14–16
Systemic plant responses, i.e., the induction of resistance in yet undamaged plant parts, therefore are generally believed to be mediated by signaling molecules that move within the plant body. Recently, however, we and another group demonstrated that HI-VOCs can be both required and sufficient to elicit defensive responses. By mechanically clipping sagebrush leaves and bagging damaged twigs, Karban and coworkers17 found that air flow from damaged to undamaged parts was required to elicit resistance in yet undamaged parts of the same plant. While Karban et al.17 focused on net plant damage, we made use of the existence of both EFN and HI-VOCs in the same plant species, Lima bean (Phaseolus lunatus), in order to directly study a single defensive response. EFN secretion by Lima bean responds to mechanical damage and herbivore feeding and can be induced also by exogenous application of JA.18 HI-VOCs can both induce19 and prime20 EFN secretion by undamaged Lima bean plants, thus causing a plant-plant communication phenomenon.
Since Baldwin and Schultz in 1983 for the first time reported ‘plant-plant communication’,21 it has been controversially debated whether or not this phenomenon plays a rolein nature.22 One question usually raised was how a plant trait can be evolutionarily stable that serves the neighboring at the cost of the volatile-emitting plant. The usual explanation was plants emit HI-VOCs as a means of indirect defense, and their neighbors only ‘eavesdrop’ on this information on the status of attack of a plant. Interestingly, plants can also be ‘primed’ by HI-VOCs, i.e., their response to herbivore attack can be much more pronounced when they before have been exposed to HI-VOCs.20,23–25
The observations by Karban et al.17 and our most recent study26 now demonstrate a new function of HI-VOCs. We exposed Lima bean plants growing naturally in the field in Mexico to beetle-damaged tendrils and observed a reduced degree of herbivory and increased growth rates of exposed as compared to control tendrils. EFN secretion can be induced by HI-VOCs released from damaged Lima bean tendrils19 and was likely the major reason of this effect. In consecutive studies, we damaged only some leaves (hereinafter called ‘emitters’) and quantified EFN secretion by other, yet undamaged leaves (‘receivers’) of the same shoot and of other, neighboring shoots. By bagging the emitter leaves with plastic foil or leaving them unbagged we could distinguish among responses of receiver leaves that were either exposed or not exposed to HI-VOCs released from the damaged leaves. Undamaged leaves increased their EFN secretion significantly when exposed to HI-VOCs of the neighboring leaves. This observation could be repeated in a second, independent experiment using beetle-damaged leaves and moving the air from those leaves towards—or away from—undamaged receivers by means of tubes and ventilators. Since damaged emitter and undamaged receiver leaves inserted on the same shoot, any plant- internal signal produced in the damaged leaves and then transported via the plant sap should have affected the undamaged receivers in all cases. That the receivers responded only when being exposed to the volatiles released from the emitters demonstrates that HI-VOCs released by damaged leaves can be a signal inducing a defensive response in yet undamaged leaves of the same plant.
HI-VOCs exhibit a hormone-like function in within-plant signaling, and plant-plant communication appears to result from plants being ‘eavesdropping’ on what is within-plant signaling worn on the outside. In spite of this potential ‘information parasitism’, the benefits of within-plant signaling mediated by external, volatile compounds are manifold. Individual HI-VOCs identified so far to cause priming or induction of defense in undamaged plants include (3Z)-hex-3-enyl acetate19 and several structurally related C6-volatiles,23,27–30 i.e., the substances involved in this phenomenon are typical green leaf volatiles and thus are released within minutes after tissue disruption. HI-VOCs-mediated within-plant signaling might thus be much faster in eliciting a systemic response than any signal that is transported as classical hormone in phloem or xylem. Moreover, herbivores often are highly mobile and their movements among plant parts do not necessarily follow the anatomy of the plant. Particularly in a liana such as Lima bean, a plant-internal signal would be less efficient as long as it is transported through the shoots, since leaves located most closely in space might insert on different shoots and therewith at an anatomical distance of several meters. VOCs can serve as a cue to elicit EFN secretion in exactly those parts of another—or even the same—plant individual, where resistance actually is required: in the spatially (yet not necessarily anatomically) neighboring parts (Fig. 1).
Figure 1.
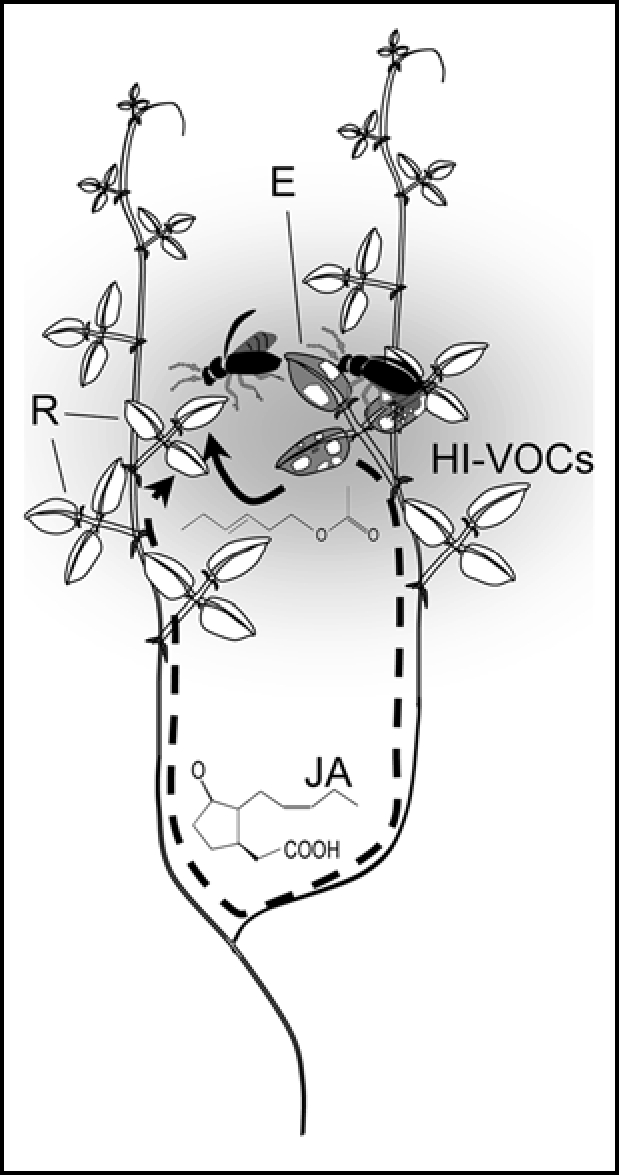
Within-plant signaling by volatiles. Volatiles (shadowed area) fill the aerial space around an herbivore-damaged emitter leaf(E, marked grey) and can rapidly induce spatially neighboring receiver leaves (R), which might anatomically be very distant. Volatile-mediated signaling (bold arrow) that functions via green leaf volatiles such as the displayed (Z)-3-hexenyl acetate thus allows much shorter signaling ways from emitter to receiver leaf that molecules such as jasmonic acid (JA) which move within the plant veins (scattered arrow).
Studies into systemic responses of plants to pathogens have so far not controlled air flow among plant parts and thus could not find the phenomenon as described by Karban et al.17 and in our study.26 Future studies must investigate to which degree herbivore or pathogen-induced volatile compounds are involved in the systemic responses of plants to local attacks. From the hypothesis given above we conclude that volatiles should be more important as resistance-eliciting means in cases of highly mobile attackers and less important in cases of attackers that only move on the plant surface or within the plant body.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=4151
References
- 1.Sticher L, Mauch-Mani B, Métraux JP. Systemic acquired resistance. Annu Rev Phytopathol. 1997;35:235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- 2.van Loon LC. Induced resistance in plants and the role of pathogenesis-related proteins. Eur J Plant Pathol. 1997;103:753–765. [Google Scholar]
- 3.Karban R, Baldwin IT. Induced responses to herbivory. Chicago and London: University of Chicago Press; 1997. [Google Scholar]
- 4.Koptur S. Extrafloral nectary-mediated interactions between insects and plants. In: Bernays EA, editor. Insect-plant interactions. IV. Boca Raton: CRC Press; 1992. pp. 81–129. [Google Scholar]
- 5.Heil M, Greiner S, Meimberg H, Krüger R, Noyer JL, Heubl G, Linsenmair KE, Boland W. Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature. 2004;430:205–208. doi: 10.1038/nature02703. [DOI] [PubMed] [Google Scholar]
- 6.Heil M, Koch T, Hilpert A, Fiala B, Boland W, Linsenmair KE. Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proc Natl Acad Sci USA. 2001;98:1083–1088. doi: 10.1073/pnas.031563398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ness JH. Catalpa bignonioides alters extrafloral nectar production after herbivory and attracts ant bodyguards. Oecologia. 2003;134:210–218. doi: 10.1007/s00442-002-1110-6. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson AG. The role of the extrafloral nectaries of Catalpa speciosa in limiting herbivory and increasing fruit production. Ecology. 1982;63:663–669. [Google Scholar]
- 9.Wäckers FL, Zuber D, Wunderlin R, Keller F. The effect of herbivory on temporal and spatial dynamics of foliar nectar production in cotton and castor. Ann Bot. 2001;87:365–370. [Google Scholar]
- 10.De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. [Google Scholar]
- 11.Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 12.Turlings TCJ, Loughrin JH, McCall PJ, Röse USR, Lewis WJ, Tumlinson JH. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA. 1995;92:4169–4174. doi: 10.1073/pnas.92.10.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Métraux JP, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B. Increase in salicylic acid at the onset of systemic acquired resistance. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- 14.Boland W, Hopke J, Donath J, Nüske J, Bublitz F. Jasmonic acid and coronatin induce odor production in plants. Angew Chemie Intl Ed. 1995;34:1600–1602. [Google Scholar]
- 15.Farmer EE, Alméras E, Krishnamurthy V. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol. 2003;6:372–378. doi: 10.1016/s1369-5266(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 16.Major IT, Constabel CP. Insect regurgitant and wounding elicit similar defense responses in Poplar leaves. Plant Signaling and Behavior. 2007;2:e1–e3. doi: 10.4161/psb.2.1.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karban R, Shiojiri K, Huntzinger M, McCall AC. Damage-induced resistance in sagebrush: Volatiles are key to intra- and interplant communication. Ecology. 2006;87:922–930. doi: 10.1890/0012-9658(2006)87[922:drisva]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Heil M. Induction of two indirect defenses benefits Lima bean (Phaseolus lunatus, Fabaceae) in nature. J Ecol. 2004;92:527–536. [Google Scholar]
- 19.Kost C, Heil M. Herbivore-induced plant volatiles induce an indirect defense in neighboring plants. J Ecol. 2006;94:619–628. [Google Scholar]
- 20.Heil M, Kost C. Priming of indirect defenses. Ecol Lett. 2006;9:813–817. doi: 10.1111/j.1461-0248.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin IT, Schultz JC. Rapid changes in tree leaf chemistry induced by damage: Evidence for communication between plants. Science. 1983;221:277–279. doi: 10.1126/science.221.4607.277. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 23.Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA. 2004;101:1781–1785. doi: 10.1073/pnas.0308037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessler A, Halitschke R, Diezel C, Baldwin IT. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia. 2006;148:280–292. doi: 10.1007/s00442-006-0365-8. [DOI] [PubMed] [Google Scholar]
- 25.Ton J, D'Allesandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]
- 26.Heil M, Silva Bueno JC. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA. 2007 doi: 10.1073/pnas.0610266104. (publ. online 7. Mar 2007: doi:10.1073/pnas.0610266104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bate NJ, Rothstein SJ. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998;16:561–569. doi: 10.1046/j.1365-313x.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 28.Farag MA, Fokar M, Zhang HA, Allen RD, Paré PW. (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. Planta. 2005;220:900–909. doi: 10.1007/s00425-004-1404-5. [DOI] [PubMed] [Google Scholar]
- 29.Ruther J, Kleier S. Plant-plant signaling: Ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-Hexen-1-ol. J Chem Ecol. 2005;31:2217–2222. doi: 10.1007/s10886-005-6413-8. [DOI] [PubMed] [Google Scholar]
- 30.Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 2005;46:1093–1102. doi: 10.1093/pcp/pci122. [DOI] [PubMed] [Google Scholar]


