Abstract
Plant hormones such as auxin derivatives are likely signals during the establishment of an arbuscular mycorrhizal (AM) symbiosis. Although reports on auxin levels during AM in different plant species are contradictory, the contribution of auxins to the establishment of an AM symbiosis might be an important factor especially for the development of lateral roots which are the preferred infection sites for the fungi. In addition to evidence that different auxins could be elevated after colonization with AM fungi, there are also overlapping gene expression patterns between auxin-treated and AM-inoculated roots that provide further clues on auxin-triggered colonization events. Using an auxin-inducible promoter-reporter system it was shown that the reporter was strongly induced in AM colonized roots, although co-localization with AM fungi was not observed. Our data are discussed in frame of a model together with other plant hormones which might be involved in the AM colonization processes.
Key words: abscisic acid, arbuscular mycorrhiza, auxin biosynthesis and metabolism, gene expression, glucosinolates, indole-3-butyric acid, lateral roots
IntroductIon
The complex relationship between host roots and AM fungi requires a continuous exchange of signals, which results in the proper development of the symbiosis.1,2 Plant hormones are signal molecules known to regulate many developmental processes in plants and are therefore suitable candidates to function in the colonization process.3,4 However, the literature on auxin levels during AM shows controversary results. There are several examples for an increase in auxins after inoculation of roots with AM fungi such as maize5,6 or soybean.7 In maize mainly indole-3-butyric acid (IBA) increased,5,8 whereas indole-3-acetic acid (IAA) remained unaltered.8,9 In soybean IAA levels were higher in AM roots than in controls.7 However, no changes in IAA levels were observed in leek and tomato.10,11 Auxins, in particular IBA, may facilitate the colonization of a host by increasing the number of lateral roots as preferential colonization sites for the fungi during early growth phases.5 In accordance with this hypothesis the most active mycelium, characterized by fungal enzyme activities, was found in newly formed lateral roots.12 The increase in lateral root development in maize roots colonized by AM fungi coincided with an increased IBA level and the phenotype of mycorrhizal maize roots could be mimicked by exogenously applying IBA to non-mycorrhizal roots.5 Addition of an inhibitor of IBA-induced root growth and lateral root induction reduced endogenous free IBA and the percentage of colonization in mycorrhizal roots.5 To further elucidate the role of different auxin derivatives we have chosen additional plant species as model organisms.
Auxin Levels and Auxin Biosynthesis Are Changed During Am Formation
Tobacco plants transformed with the auxin inducible promoter GH3 fused to the GUS reporter gene were used to investigate whether AM structures co-localize in cells indicative of auxin accumulation. Although the GUS activity increased significantly in AM inoculated roots, there was no obvious correlation between GUS expression patterns and fungal structures.13
Nasturtium (Tropaeolum majus L.) is an interesting species in which to study the relationship between auxin and glucosinolate (GSL) metabolism as well as the role of these compounds during symbiosis, due to its relative simple GSL pattern compared to other plant species.14 Despite the fact that high levels of benzylglucosinolate are present in roots of nasturtium, the colonization rate with different AM fungal species was comparable to non-GSL plant species.14 We have shown that the levels of three auxins native to this plant15 change during early stages of AM colonization, but with different patterns.13 Benzylglucosinolate, the main GSL of T. majus was also increased during AM colonization.14 Very early free IAA and IBA were lower in infected than control roots, whereas phenylacetic acid (PAA) levels were higher in infected roots than in controls. At later stages PAA was reduced in colonized roots, whereas especially IBA was increased compared to controls.13 Even though many microorganisms synthesis auxins, in hyphae of Glomus intraradices none of the above auxins were detectable, although Glomus spores contained minute amounts of IAA.8 The biosynthesis routes for IAA, IBA and PAA were investigated using heavy labeled isotopes as precursors in control and AM-inoculated nasturtium roots. While not much difference was found in the IAA labeling pattern between controls and AM inoculated roots at both time points, IBA synthesis was slightly higher in AM roots. Double labeling experiments showed that two distinct pathways, a tryptophan-dependent and a tryptophan-independent biosynthetic pathway contributed to the synthesis of IAA in T. majus roots but neither was preferentially induced during AM colonization.13
Auxin-and AM-Induced Gene Expression
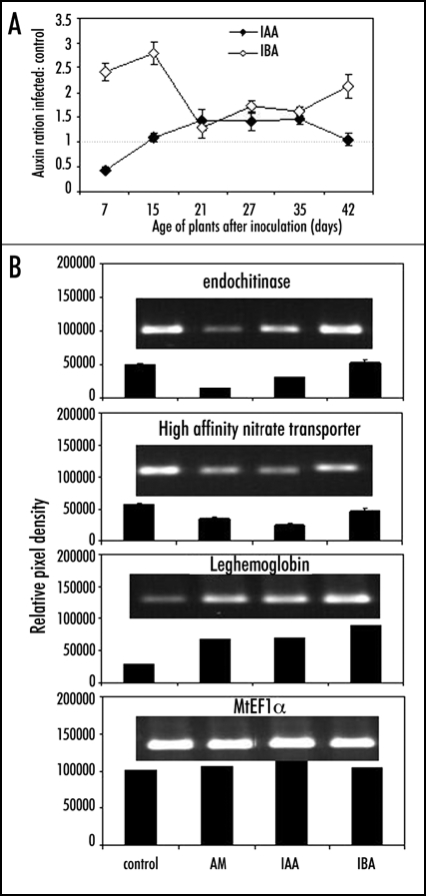
The mycorrhization of roots of the model legume Medicago truncatula resulted in increased levels of IBA (Fig. 1). This increase was already visible prior to detectable intraradical structures and continued up to 42 days after inoculation. A slight transient increase of IAA was observed at the onset of AM structures in the roots. For several genes an overlap in the expression after treatment with IBA and AM colonization was found (Fig. 1). A transcript for leghemoglobin was upregulated in AM colonized roots as well as after IAA and IBA treatment. Leghemoglobins have high affinity for oxygen and play an important role during N2 fixation in the Rhizobium-legume interaction.16 Although their function during AM symbiosis is not yet clear, increased expression of the leghemoglobin gene VfLb29 from Vicia faba was also demonstrated in AM inoculated roots.17 Contrary, a transcript for a high affinity nitrate transporter was downregulated in AM roots and after treatment with both auxins. However, since nitrate transporters are present in large gene families, differential expression of individual transporters is highly likely and other members of the family might respond different. A member of an endochitinase family was down-regulated by mycorrhizal colonization and IAA treatment but not affected after application of IBA to Medicago roots, indicating that the control of the defense response is important. This could be an example where the two auxins regulate different plant responses. Together, these results suggest that increased auxin levels and subsequent auxin-induced gene expression might contribute to the phenotypical changes during mycorrhizal colonization (Fig. 2).
Figure 1.
(A) Free IAA and IBA levels during AM development in Medicago truncatula given as ratio infected to control roots. All values above 1 (dotted line) indicate an increase in AM roots compared to controls. (B) Auxin and AM-induced gene expression of selected genes identified by microarray analysis monitored in Medicago truncatula. The elongation factor EF1α was used as housekeeping gene. Free auxins were determined as previously described.13 Total RNA was prepared using the TRIZOL® (Invitrogen, Carlsbad, CA, USA) method according to the manufacturer's instructions from 100 mg fresh weight of each sample. RT-PCR was carried out with the following primers: MtEF1α sense 5′-CAATGTGAGAGGTGTGGCAAT C, antisense 5′-GGAGTGAAGCAGATGATCTGTTG; MtLeghem sense 5′AGAGAAATTGGGTTTCACAGAGA, antisense 5′-TAACTCATTGCTTTCTTAATTGCAC; MtHANT sense 5′-TCGGAATGCGAGGAAGATTA, antisense 5′-AAAGCATGGGAAATTCATTAGC; MtChit sense 5′-GTAACGGTCAAACCCCTGAA, antisense 5′-CGCACTATGGGGGATAAAAG.
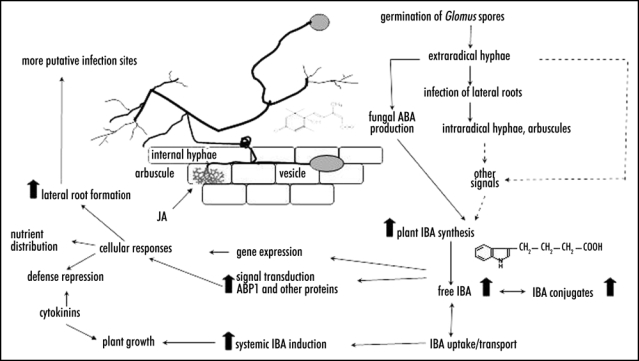
Figure 2.
A model for hormone action in arbuscular mycorrhizal symbiosis. The model is a compilation from different plant species. It is based on the model previously proposed by Kaldorf and Ludwig-Müller,5 incorporating findings from the present study and other investigations.6,7,13,18,22 More explanations are given in the text.
A Self Regulatory Infection Cycle?
Various plant species react with an increase of auxins to inoculation with AM fungi. In maize it was shown that IBA is the major auxin induced during AM colonization. In addition to free IBA conjugate formation was also observed (Fig. 2).6,13 IBA levels can be induced by abscisic acid (ABA) as demonstrated in maize.18 Since Glomus intraradices contains considerable amounts of ABA in hyphae, the AM fungus might control IBA biosynthesis of its host via hormonal signals. This is in accordance with increased ABA levels in AM inoculated roots of maize,19 nasturtium and Medicago truncatula. The auxin signal is then transferred via signalling components, for example the auxin binding protein ABP120 which can be induced by IBA and AM in maize.8 This signal then leads to auxin-induced or -repressed gene function necessary for AM establishment. Together with other hormones such as cytokinins and jasmonate21,22 this will result in an increased growth promotion of inoculated plants and/or the development of the fungus. Finally, the increased auxin levels lead to the formation of more lateral roots which constitute preferential penetration sites for the AM hyphae, thus closing the infection cycle. Future research has to provide functional proof for these hypotheses.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=4152
References
- 1.Gianinazzi-Pearson V. Plant cell responses to arbuscular mycorrhizal fungi: Getting to the roots of the symbiosis. Plant Cell. 1996;8:1871–1883. doi: 10.1105/tpc.8.10.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hause B, Fester T. Molecular and cell biology of arbuscular mycorrhizal symbiosis. Planta. 2005;221:184–196. doi: 10.1007/s00425-004-1436-x. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig-Müller J. Hormonal balance in plants during colonization by mycorrhizal fungi. In: Douds DD, Kapulnik Y, editors. Arbuscular Mycorrhizas: Physiology and function. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 263–285. [Google Scholar]
- 4.Barker SJ, Tagu D. The roles of auxins and cytokinins in mycorrhizal symbiosis. J Plant Growth Regul. 2000;19:144–154. doi: 10.1007/s003440000021. [DOI] [PubMed] [Google Scholar]
- 5.Kaldorf M, Ludwig-Müller J. AM fungi might affect the root morphology of maize by increasing indole-3-butyric acid biosynthesis. Physiol Plant. 2000;109:58–67. [Google Scholar]
- 6.Fitze D, Wiepning A, Kaldorf M, Ludwig-Müller J. Auxins in the development of an arbuscular mycorrhizal symbiosis in maize. J Plant Physiol. 2005;162:1210–1219. doi: 10.1016/j.jplph.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Meixner C, Ludwig-Müller J, Miersch O, Gresshoff P, Staehelin C, Vierheilig H. Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta. 2005;222:709–715. doi: 10.1007/s00425-005-0003-4. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig-Müller J, Kaldorf M, Sutter EG, Epstein E. Indole-3-butyric acid (IBA) is enhanced in young maize (Zea mays L.) roots colonized with the arbuscular mycorrhizal fungus Glomus intraradices. Plant Sci. 1997;125:153–162. [Google Scholar]
- 9.Schmitz O, Danneberg G, Hundeshagen B, Klingner A, Bothe H. Quantification of vesicular-arbuscular mycorrhiza by biochemical parameters. J Plant Physiol. 1991;139:106–111. [Google Scholar]
- 10.Torelli A, Trotta A, Acerbi L, Arcidiacono G, Berta G, Branca C. IAA and ZR contant in leek (Allium porrum L.), as influenced by P nutrition and arbuscular mycorrhizae, in relation to plant development. Plant Soil. 2000;226:29–35. [Google Scholar]
- 11.Shaul-Keinan O, Gadkar V, Ginzberg I, Grünzweig JM, Chet I, Elad Y, Wininger S, Belausov E, Eshed Y, Atzmon N, Ben-Tal Y, Kapulnik Y. Hormone concentrations in tobacco roots change during arbuscular mycorrhizal colonization with Glomus intraradices. New Phytol. 2002;154:501–508. doi: 10.1046/j.1469-8137.2002.00388.x. [DOI] [PubMed] [Google Scholar]
- 12.Tisserant B, Gianinazzi S, Gianinazzi-Pearson V. Relationships between lateral root order, arbuscular mycorrhiza development, and the physiological state of the symbiotic fungus in Platanus acerifolia. Can J Bot. 1996;74:1947–1955. [Google Scholar]
- 13.Jentschel K, Thiel D, Rehn F, Ludwig-Müller J. Arbuscular mycorrhiza enhances auxin levels and alters auxin biosynthesis in Tropaeolum majus during early stages of colonization. Physiol Plant. 2007;129:320–333. [Google Scholar]
- 14.Vierheilig H, Bennett R, Kiddle G, Kaldorf M, Ludwig-Müller J. Differences in glucosinolate patterns and arbuscular mycorrhizal status of glucosinolate-containing plant species. New Phytol. 2000;146:343–352. doi: 10.1046/j.1469-8137.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig-Müller J, Cohen JD. Identification and quantification of three active auxins in different tissues of Tropaeolum majus. Physiol Plant. 2002;115:320–329. doi: 10.1034/j.1399-3054.2002.1150220.x. [DOI] [PubMed] [Google Scholar]
- 16.Heidstra R, Nilsen G, Martinez-Abarca F, van Kammen A, Bisseling T. Nod factor-induced expression of leghemoglobin to study the mechanism of NH4NO3 inhibition on root hair deformation. Mol Plant Microbe Interact. 1997;10:215–220. doi: 10.1094/MPMI.1997.10.2.215. [DOI] [PubMed] [Google Scholar]
- 17.Frühling M, Roussel H, Gianinazzi-Pearson V, Pühler A, Perlick AM. The Vicia faba leghemoglobin gene VfLb29 is induced in root nodules and in roots colonized by the arbuscular mycorrhizal fungus Glomus fasciculatum. Mol Plant-Microbe Interact. 1997;10:124–131. doi: 10.1094/MPMI.1997.10.1.124. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig-Müller J, Schubert B, Pieper K. Regulation of IBA synthetase by drought stress and abscisic acid. J Exp Bot. 1995;46:423–432. [Google Scholar]
- 19.Danneberg G, Latus C, Zimmer W, Hundeshagen B, Schneider-Poetsch HJ, Bothe H. Influence of vesicular-arbuscular mycorrhiza on phytohormone balances in maize (Zea mays L.) J Plant Physiol. 1992;141:33–39. [Google Scholar]
- 20.Christian M, Steffens B, Schenck D, Burmester S, Böttger M, Lüthen H. How does auxin enhance cell elongation? Roles of auxin-binding proteins and potassium channels in growth control. Plant Biol. 2006;8:346–352. doi: 10.1055/s-2006-923965. [DOI] [PubMed] [Google Scholar]
- 21.Ginzberg I, David R, Shaul O, Elad Y, Winiger S, Ben-Dor B, Badani H, Fang Y, van Rhijn P, Li Y, Hirsch AM, Kapulnik Y. Glomus intraradices colonization regulates gene expressin in tobacco roots. Symbiosis. 1998;25:145–157. [Google Scholar]
- 22.Hause B, Maier W, Miersch O, Kramell R, Strack D. Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol. 2002;130:1213–1220. doi: 10.1104/pp.006007. [DOI] [PMC free article] [PubMed] [Google Scholar]