Abstract
Root hairs cells are highly polarized cellular structures resulting from tip growth of specific root epidermal cells. Root-hair morphogenesis involves many aspects regulating tip growth such as exocitosis, ion flux, calcium homeostasis, reactive oxygen species (ROS), and cytoskeleton. These cells are excellent models for studying polar growth and can be challenged with many extracellular factors affecting the pattern of growth named Nod factors, elicitors, hormones, etc. The general scenery is that the well described tip-high intracellular Ca2+ gradient plays a central role in regulating tip growth. On the other hand, ROS plays a key role in various processes, for example hypersensitive response, root hair development, hormone action, gravitropism and stress responses. However, ROS has recently emerged as a key player together with calcium in regulating polar growth, not only in root hair cells but also in pollen tubes, filamentous fungi and fucoid cells. Furthermore, Ca2+-permeable channel modulation by ROS has been demonstrated in Vicia faba guard cells and Arabidopsis root hairs. Recently, root hair cells were shown to experiment ROS, pH and calcium oscillations coupled to growth oscillation. These recent findings allow considering that root hair cells present a similar pattern of growth as described for pollen tubes.
Key words: root hairs, polar growth, calcium, reactive oxygen species, cell wall, NADPH oxidase
Polar Growth Depends on Ionic and Molecular Gradients Located at the Tip
Root hairs are highly polarized cellular structures resulting from the growth of specific root epidermal cells. These tubular root cells extend by tip growth and play an important role in sensing extracellular biotic and abiotic conditions, including nutritional status.1 Root hairs are easy to grow and visualize under laboratory conditions and can be challenged with many extracellular factors that affect the pattern of growth, including Nod factors, elicitors and hormones. The morphogenesis of these cells involves the interaction of many processes, including vesicle exocytosis, calcium homeostasis, localized production of reactive oxygen species (ROS), and modification of the cytoskeleton.2–5 A tip-focused free cytosolic Ca2+ gradient ([Ca2+]c) plays a central role in regulating tip growth by facilitating vesicle exocytosis.6 In addition, the production and accumulation of ROS is known to regulate calcium homeostasis through modulation of Ca2+ permeable channels.7
Root hair cells, as well as Fucoid cells, pollen tubes and fungal hyphae possess a typical tip growth intracellular organization that is highly polarized, with growth limited to the apical dome,8 which contains elevated tip-focused calcium ion levels. Since this pattern of growth strictly requires exocytosis in the apical region, calcium ions and the actin cytoskeleton have been proposed as key players in the fine regulation of this process.9,10 Root hairs and pollen tubes display the well-described reverse fountain-like streaming,11 which relies on actin cytoskeleton remodeling. This pattern involves upward cytoplasmic streaming from the periphery of the root hair tube to the subapical region, and downward cytoplasmic streaming through the center of the root hair tube. Vesicles are delivered to the subapex, where the streaming reverses direction.
The organization of actin microfilaments in root hairs has been visualized by microinjecting fluorescent phalloidin, a fungal toxin that binds to filamentous actin, or by expression of actin binding domains fused to GFP.12–14 In root hairs, long actin bundles have been observed to run more or less longitudinally through the root hair tube, and the nucleus seems to be surrounded with microfilaments.12 However, close to the tip, the long actin bundles flare out into a network of fine bundles.12,15 It has long been established that actin polymerization can generate forces that contribute to outgrowth of the plasma membrane and intracellular movements, such as those observed during Listeria infection or endosome trafficking.16–19 G-actin foci have been reported in the tip region of root hairs and pollen tubes,17,20 implying that actin polymerization at the tip could play an important role in supporting polar growth. The increased tip concentration of G-actin could fuel the continuous polymerization required for polarized growth in this zone and could result from actin remodeling by calcium and pH-sensitive actin-binding proteins, such as ADF, gelsolin, villin and others.
Calcium Gradient, Proton Flux and ROS Production Regulates Polar Growth in Root Hair Cells
Root hair cells, like other tip-growing cells such as pollen tubes, fucoid cells and hyphae, represent a classic example of polarized cell growth. This is clearly characterized by a substantial accumulation of cytoplasm in the tip region, which suggests that the growth precursors accumulate in the tip; in fact, most of the factors involved in cell growth have been found to be localized to this region.
The tip-focused cytosolic Ca2+ gradient in growing root hairs has been widely considered as a master regulatory mechanism controlling polar growth, similar to other tip-growing cells, and failure to maintain this gradient results in growth inhibition.6 The [Ca2+]c has been shown to modulate many processes involved in the regulation of exocytosis, as well as to finely tune calcium-regulated proteins that have profound effects on the organization of the actin cytoskeleton.9,21–23
While calcium is thought to result in stiffening of the cell wall, protons are believed to cause softening, promoting cell wall plasticity and extensibility.24 Thus, a relationship between Ca2+ and pH can be established. Arabidopsis root hair cell walls have been shown to acidify after the root hair initiation site has been determined, but not before. Interestingly, this extracellular acidification is accompanied by a corresponding cytoplasmic alkalinization.25 This suggests that the initiation and elongation of root hairs represent two different stages of growth that might require different factors.25 These pH measurements have been made using pH sensitive dye, such as Oregon Green fused to a cellulose binding domain to measure the pH of the cell wall compartment.25,26 The use of pH-sensitive GFP constructs targeted to the cell wall should allow for pH measurements in compartments that are inaccessible to chemically-generated fluorescent probes.27 Recently, a pH sensitive GFP derived from the orange seapen (Ptilosarcus gurneye) was demonstrated to be a reliable indicator of cellular pH when expressed in bacteria and plants.28 This new in vivo pH probe offers several advantages over previous probes used for this purpose, including a better dynamic range and superior stability under neutral and acidic pH conditions, making it suitable for apoplastic and cytoplasmic measurements.
Intracellular and extracellular ROS play key roles in regulating a myriad of cellular responses, including: the hypersensitive response, programmed cell death, development, gravitropism, hormonal signaling, stomata opening and ion channel regulation.29 Plant NADPH oxidases are membrane bound proteins that constitute key enzymatic components involved in the generation of superoxide radicals.7,30–33 The idea that ROS levels are important for sustaining polar growth was clearly supported by the findings of Foreman et al., (2003). These studies showed that an Arabidopsis NADPH oxidase mutant fail to sustain root hair cell elongation, and mutant root hair cells are inhibited at the outward bulging stage. Interestingly, these mutants were unable to accumulate ROS at the tip of root hair cells and the Ca2+ gradient was absent. However, addition of extracellular ROS rescued the phenotype and favored cell elongation.7 Evidence for the role of ROS in sustaining polarized growth was strengthened by the fact that ROS can activate the opening of calcium channels, as demonstrated by patch-clamp studies.7 Finally, the apical localization of ROS in the tip region also suggested a potential mechanism by which cell wall properties are regulated.
Other Tip-Growing Cells also Require ROS Production to Sustain Polarized Growth
Recent work by Potocky et al., (2007)34 showed that pollen tube cells require the activity of NADPH oxidase to sustain pollen tube growth. Since NADPH oxidase generates extracellular ROS by transferring electrons from cytosolic NADPH across the plasma membrane to reduce molecular oxygen, with the consequent production of the superoxide, the authors used nitroblue tetrazolium (NBT) in the medium as an indicator of extracellular superoxide. Using this approach, they found that the apical region of the pollen tube displays clear accumulation of ROS.34 Both DPI, an inhibitor of NADPH oxidase, and TMPP, a ROS scavenger, inhibited pollen tube growth, suggesting an essential role for ROS in supporting growth.34 Furthermore, the polarization of Fucus serratus zygotes, a well-described model system in which photopolarization have been studied, the polarization involves the generation of a Ca2+ gradient. Since these cells also form well-defined ROS gradients in the tip region, and this depends on the presence of a Ca2+ gradient,35,36 the authors suggest that ROS in the tip region stimulate the generation of a calcium gradient.
In Aspergillus nidulans, a hyphal fungus in which apical dominance results in the growing hyphal tip, localized apical accumulation of ROS has been also established.37 Thus, a correlation between localized production of ROS and reinforcement of apical dominance has been suggested in these tip-growing cells. Furthermore, evidence suggests that NADPH oxidases (Nox) or related flavoproteins are responsible for this ROS-generating mechanism, which determines a polarity axis at hyphal tips.37
Finally, ROS levels in tip-growing cells could somehow be modulated by ROS changes in other subcellular locations. For instance, ROS accumulation can be observed in endomembranes and mitochondria.38,39 Mitochondria have been given special attention since they exhibit high levels of ROS production, and failure to control mitochondrial ROS levels can induce programmed cell death.40,41 It is also intriguing that isolated nuclei from BY-2 cells are capable of producing ROS in a calcium-dependent manner when treated with cryptogein, a proteinaceous elicitor derived from the pathogenic fungus Phytophthora cryptogea.39 This result indicates that the plant nucleus can participate in ROS signaling as demonstrated in animal cells, and this could significantly affect gene expression.42
New Findings in Polarized Growth: Ca2+, pH and ROS Act in Concert to Sustain Growth Oscillations
Pollen tubes are known to experience oscillatory growth, which is characterized by episodes of fast growth followed by periods of slower growth.43 In order to achieve this pattern, the factors involved in pollen tube growth function in concert to maintain the periodic oscillations. For example, cytoplasmic Ca2+ levels follow a pattern of oscillation during which the highest concentration follows the highest peak in growth.43 Many other factors are also found to oscillate in this manner, including protons, pH and NADPH.44,45 In addition, the activity of proteins such as ADF (actin depolymerizing factor) undergo cycles of activation that are likely related to actin polymerization.21,46
These oscillatory components were unknown until the recent report by Monschausen et al., (2008)48 that described oscillatory growth in Arabidopsis root hair cells. This was the first work to describe growth oscillations similar to those found in pollen tubes.47,48 Root hair cells grow at a slower rate than pollen tubes, 0.9–3.2 µm/min compared to 0.2–0.5 µm/sec. Nevertheless, they both exhibit the same oscillatory pattern during growth. Another significant finding of this work is that Ca2+ oscillations lag the maximum peak in growth. These results are similar to those described for pollen tubes, not in terms of timing, but with regard to the sequence of events.
Another study showed that oscillations in growth correlate with similar changes in extracellular pH and ROS, which allow for modulation of tip growth in Arabidopsis root hairs.47 It is interesting to note that the peak in pH and ROS levels follows tip growth. These results are significant, since cell wall pH and ROS have been reported to affect cell wall expansion. The authors suggest that increases in pH and ROS could play pivotal roles in restricting tip growth by locally strengthening the cell wall. This hypothesis is supported by the finding that an increase in the pH of the medium results in growth inhibition of root hair cells, while decreasing the pH results in cell bursting as a consequence of uncontrolled growth.47 Cell bursting is also observed when an NADPH oxidase inhibitor, such as DPI, is added to the medium. Under these conditions, the root hair cells probably burst due to reduced ROS production and an inability to sustain cell wall remodeling.47
Mutations in the NADPH oxidase of Arabidopsis (rhd2) result in an inability of root hair cells to switch from the bulging out stage to the tip-growing stage.7 However, when the rdh2 mutant is grown in a high-pH environment, the root hair cells are able to elongate, suggesting that ROS production by NADPH oxidase is not absolutely necessary for root hair elongation.47 However, these cells do not experience growth and ROS oscillations, suggesting that the ROS-generating enzyme is required for regulation of the oscillatory processes.
Recently, root hair cells were shown to display a tip-focused ROS distribution based on pseudoratiometric analysis.49,50 This ROS distribution is very sensitive to the presence of NADPH oxidase inhibitors (DPI), which inhibit the formation of this gradient and tip growth, and thus NADPH oxidase has been suggested to play a role in generating the apical ROS gradient. In addition, localized ROS accumulation is thought to play a key role in signal transduction since the addition of external fungal elicitors, ROS, UV radiation and Nodulation factors induce specific changes in intracellular ROS levels.49 In fact, the induced ROS changes resemble Ca2+ changes induced in response to nodulation factors, underscoring the proposed connection between ROS and Ca2+.
The previously described results raise questions regarding the mechanism of calcium channel regulation in the tip region. For instance, ROS was shown to regulate calcium channels,7 and this mechanism could generate a positive feedback loop that continuously stimulates apical calcium channels.51 However, the NADPH oxidase mutant rhd2 can be rescued by increasing the extracellular pH, suggesting an additional mechanism of calcium channel activation that contributes to the generation of apical calcium gradients and ROS production.
Mechanisms Regulating ROS Production in Tip-Growing Cells
While the evidence to date indicates a critical role for apical ROS levels in tip growth, two factors must be considered when describing their function: first, the role that ROS accumulation might play at the extracellular level, where cell wall remodeling could be the main target; and second, that intracellular ROS levels could have other, indirect effects, for instance in the regulation of Ca2+ channels. It seems plausible that there are several mechanisms coupled to ROS production. Unlike mammalian NADPH oxidase, plant homologs have hydrophilic N-terminal regions containing two EF-hand motifs, suggesting that their activation is dependent on Ca2+.52,53 However, the importance of NADPH oxidase Ca2+ binding for ROS production is still poorly understood. Nevertheless, this association may provide fine-tuning of the regulation of ROS-producing enzymes, and suggests a close relationship between calcium ions and ROS production. Addition of ionomycin, a Ca2+ ionophore, activates NADPH oxidase and increases ROS production.54 On the other hand, Arabidopsis NADPH oxidase can be directly phosphorylated in vivo, and Ca2+ binding and phosphorylation have the ability to synergistically activate the ROS-producing enzyme.54,55
Other factors besides calcium are also important for regulating ROS levels in plant cells. Small GTPases, such as Rop family proteins, play a pivotal role in ROS production in Arabidopsis root hair cells.33,56,57 Constitutive expression of Rop delocalizes the apical Ca2+ gradient and induces isotropic growth at the tip region of Arabidopsis root hair cells.57 Another example of small GTPase-dependent regulation has been described in rice, where NADPH oxidase can be regulated by the binding of Rac GTPase to its N-terminal extension.56 It has been recently shown that cellular ATP has the capacity to induce ROS changes in Arabidopsis and Medicago root hair cells,58–60 a finding that has also been observed in Phaseolus vulgaris root hair cells.49 These results reinforce the idea that ATP could be an important signaling molecule, with the ability to regulate ROS levels. Finally, hydrogen peroxide homeostasis results from the balance between the regulation of enzymes that increase ROS levels and the activity of ROS scavengers. In fact, catalases can be finely regulated by calcium and calmodulin,61 suggesting that these activators could comprise a regulatory mechanism for ROS production and scavenger activity.
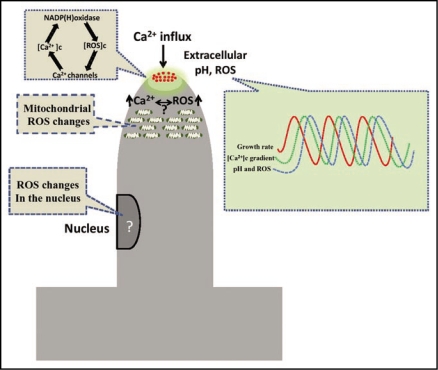
The current model for the role of ROS in tip growth is summarized in Figure 1. The role of ROS in supporting plant growth at the tip dome and the mechanism involved in the feedback responsible for ROS and calcium modulation are also described. We also show how recently reported pH and ROS oscillations in the tip region are coupled to growth patterns. Finally, we depict the recently reported findings describing intracellular ROS changes in the mitochondrial and nuclear compartments. While the evidence suggests a primary role for ROS in regulating polarized growth, many other aspects involved in determining growth polarity remain to be addressed. The development of new tools for monitoring superoxide production in vivo (that is, the products generated by NADPH oxidase activity) using molecular probes remains a future challenge. It is worth mentioning that a circularly permuted yellow fluorescent protein (cpYFP) that has been widely used as a calcium sensor was recently reported as a novel biosensor for O2.−, and has been successfully used to measure flashes of superoxide production in mitochondria.62 This type of probe will allow for a myriad of experiments that will facilitate elucidation of the spatial and temporal resolution of ROS production not only in root hair cells, but also in many other animal and plant cells in which ROS plays a potential role.
Figure 1.
Model that depicts the factors involved in polar growth. Calcium fluxes at the tip as well as pH and ROS changes are associated with polar growth. Right inset depicts a model where the generated extracellular ROS, pH as well as intracellular [Ca2+]c are coupled to growth oscillations. Left inset illustrate a putative model for the regulation of ROS and calcium production as well as other sites for ROS generation. The data on Growth, [Ca2+]c, pH and ROS oscillations were taken from Monshausen et al., (2007 and 2008)47,48 and the model on ROS, [Ca2+]c and NADPH oxidase was modified from Cardenas et al., (2008).49
Acknowledgements
This work was partially supported by grants from Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México, No. IN228903 (L.C.) and CONACyT 58323 (L.C.).
Abbreviations
- ROS
reactive oxygen species
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7341
References
- 1.Shin R, Berg RH, Schachtman DP. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005;46:1350–1357. doi: 10.1093/pcp/pci145. [DOI] [PubMed] [Google Scholar]
- 2.Schindelman G, Morikami A, Jung J, Baskin TI, Carpita NC, Derbyshire P, McCann MC, Benfey PN. COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev. 2001;15:1115–1127. doi: 10.1101/gad.879101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones DL, Blancaflor EB, Kochian LV, Gilroy S. Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ. 2006;29:1309–1318. doi: 10.1111/j.1365-3040.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 4.Sangiorgio V, Pitto M, Palestini P, Masserini M. GPI-anchored proteins and lipid rafts. Ital J Biochem. 2004;53:98–111. [PubMed] [Google Scholar]
- 5.Jones MA, Raymond MJ, Smirnoff N. Analysis of the root-hair morphogenesis transcriptome reveals the molecular identity of six genes with roles in root-hair development in Arabidopsis. Plant J. 2006;45:83–100. doi: 10.1111/j.1365-313X.2005.02609.x. [DOI] [PubMed] [Google Scholar]
- 6.Wymer CL, Bibikova TN, Gilroy S. Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 1997;12:427–439. doi: 10.1046/j.1365-313x.1997.12020427.x. [DOI] [PubMed] [Google Scholar]
- 7.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 8.Shaw SL, Dumais J, Long SR. Cell surface expansion in polarly growing root hairs of Medicago truncatula. Plant Physiol. 2000;124:959–970. doi: 10.1104/pp.124.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battey NH, Blackbourn HD. The control of exocytosis in plant cells. New Phytol. 1993;125:307–338. doi: 10.1111/j.1469-8137.1993.tb03883.x. [DOI] [PubMed] [Google Scholar]
- 10.Baluska F, Salaj J, Mathur J, Braun M, Jasper F, Samaj J, Chua NH, Barlow PW, Volkmann D. Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Developmental biology. 2000;227:618–632. doi: 10.1006/dbio.2000.9908. [DOI] [PubMed] [Google Scholar]
- 11.Sieberer B, Emons AM. Cytoarchitecture and pattern of cytoplasmic streaming in root hairs of Medicago truncatula during development and deformation by nodulation factors. Protoplasma. 2000;214:118–127. [Google Scholar]
- 12.Cárdenas L, Vidali L, Domínguez J, Pérez H, Sánchez F, Hepler PK, Quinto C. Rearrangement of actin microfilaments in plant root hairs responding to rhizobium etli nodulation signals. Plant Physiol. 1998;116:871–877. doi: 10.1104/pp.116.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voigt B, Timmers AC, Samaj J, Muller J, Baluska F, Menzel D. GFP-FABD2 fusion construct allows in vivo visualization of the dynamic actin cytoskeleton in all cells of Arabidopsis seedlings. Eur J Cell Biol. 2005;84:595–608. doi: 10.1016/j.ejcb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Kost B, Spielhofer P, Chua NH. A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 1998;16:393–401. doi: 10.1046/j.1365-313x.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- 15.Miller DD, de Ruijter NCA, Bisseling T, Emons AM. The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 1999;17:141–154. [Google Scholar]
- 16.Vidali L, McKenna ST, Hepler PK. Actin polymerization is essential for pollen tube growth. Mol Biol Cell. 2001;12:2534–2545. doi: 10.1091/mbc.12.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cárdenas L, Lovy-Wheeler A, Wilsen KL, Hepler PK. Actin polymerization promotes the reversal of streaming in the apex of pollen tubes. Cell Motil Cytoskel. 2005;61:112–127. doi: 10.1002/cm.20068. [DOI] [PubMed] [Google Scholar]
- 18.Zeile WL, Zhang F, Dickinson RB, Purich DL. Listeria's right-handed helical rocket-tail trajectories: mechanistic implications for force generation in actin-based motility. Cell Motil Cytoskel. 2005;60:121–128. doi: 10.1002/cm.20050. [DOI] [PubMed] [Google Scholar]
- 19.Voigt B, Timmers AC, Samaj J, Hlavacka A, Ueda T, Preuss M, Nielsen E, Mathur J, Emans N, Stenmark H, Nakano A, Baluska F, Menzel D. Actin-based motility of endosomes is linked to the polar tip growth of root hairs. Eur J Cell Biol. 2005;84:609–621. doi: 10.1016/j.ejcb.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 20.He X, Liu YM, Wang W, Li Y. Distribution of G-actin is related to root hair growth of wheat. Ann Bot. 2006;98:49–55. doi: 10.1093/aob/mcl084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CY, Wong EI, Vidali L, Estavillo A, Hepler PK, Wu HM, Cheung AY. The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. Plant Cell. 2002;14:2175–2190. doi: 10.1105/tpc.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun M, Hauslage J, Czogalla A, Limbach C. Tip-localized actin polymerization and remodeling, reflected by the localization of ADF, profilin and villin, are fundamental for gravity-sensing and polar growth in characean rhizoids. Planta. 2004;219:379–388. doi: 10.1007/s00425-004-1235-4. [DOI] [PubMed] [Google Scholar]
- 23.Fan X, Hou J, Chen X, Chaudhry F, Staiger CJ, Ren H. Identification and characterization of a Ca2+-dependent actin filament-severing protein from lily pollen. Plant Physiol. 2004;136:3979–3989. doi: 10.1104/pp.104.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 25.Bibikova TN, Jacob T, Dahse I, Gilroy S. Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development. 1998;125:2925–2934. doi: 10.1242/dev.125.15.2925. [DOI] [PubMed] [Google Scholar]
- 26.Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S. Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell. 2001;13:907–921. doi: 10.1105/tpc.13.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao D, Knight MR, Trewavas AJ, Sattelmacher B, Plieth C. Self-reporting Arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol. 2004;134:898–908. doi: 10.1104/pp.103.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulte A, Lorenzen I, Bottcher M, Plieth C. A novel fluorescent pH probe for expression in plants. Plant Methods. 2006;2:7. doi: 10.1186/1746-4811-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittler R, Berkowitz G. Hydrogen peroxide, a messenger with too many roles? Redox Rep. 2001;6:69–72. doi: 10.1179/135100001101536067. [DOI] [PubMed] [Google Scholar]
- 30.Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres MA, Jones JD, Dangl JL. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nature Genet. 2005;37:1130–1134. doi: 10.1038/ng1639. [DOI] [PubMed] [Google Scholar]
- 32.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Jones MA, Raymond MJ, Yang Z, Smirnoff N. NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J Exp Bot. 2007;58:1261–1270. doi: 10.1093/jxb/erl279. [DOI] [PubMed] [Google Scholar]
- 34.Potocky M, Jones MA, Bezvoda R, Smirnoff N, Zarsky V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- 35.Coelho SM, Brownlee C, Bothwell JH. A tip-high, Ca2+-interdependent, reactive oxygen species gradient is associated with polarized growth in Fucus serratus zygotes. Planta. 2008;227:1037–1046. doi: 10.1007/s00425-007-0678-9. [DOI] [PubMed] [Google Scholar]
- 36.Coelho SM, Taylor AR, Ryan KP, Sousa-Pinto I, Brown MT, Brownlee C. Spatiotemporal patterning of reactive oxygen production and Ca2+ wave propagation in fucus rhizoid cells. Plant Cell. 2002;14:2369–2381. doi: 10.1105/tpc.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semighini CP, Harris SD. Regulation of apical dominance in Aspergillus nidulans hyphae by reactive oxygen species. Genetics. 2008;179:1919–1932. doi: 10.1534/genetics.108.089318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller IM. PLANT MITOCHONDRIA AND OXIDATIVE STRESS: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Ann Rev Plant Physiol Plant Molec Biol. 2001;52:561–591. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]
- 39.Ashtamker C, Kiss V, Sagi M, Davydov O, Fluhr R. Diverse subcellular locations of cryptogein-induced reactive oxygen species production in tobacco Bright Yellow-2 cells. Plant Physiol. 2007;143:1817–1826. doi: 10.1104/pp.106.090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam E, Kato N, Lawton M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411:848–853. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- 41.Gao C, Xing D, Li L, Zhang L. Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta. 2008;227:755–767. doi: 10.1007/s00425-007-0654-4. [DOI] [PubMed] [Google Scholar]
- 42.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;8:349. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 43.Holdaway-Clarke TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell. 1997;9:1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cárdenas L, McKenna ST, Kunkel JG, Hepler PK. NAD(P)H oscillates in pollen tubes and is correlated with tip growth. Plant Physiol. 2006;142:1460–1468. doi: 10.1104/pp.106.087882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovy-Wheeler A, Kunkel JG, Allwood EG, Hussey PJ, Hepler PK. Oscillatory increases in alkalinity anticipate growth and may regulate actin dynamics in pollen tubes of lily. Plant Cell. 2006;18:2182–2193. doi: 10.1105/tpc.106.044867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cárdenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK. Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol. 2008;146:1611–1621. doi: 10.1104/pp.107.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA. 2007;104:20996–21001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monshausen GB, Messerli MA, Gilroy S. Imaging of the yellow cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008;147:1690–1698. doi: 10.1104/pp.108.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cárdenas L, Martinez A, Sanchez F, Quinto C. Fast, transient and specific intracellular ROS changes in living root hair cells responding to Nod factors (NFs) Plant J. 2008 doi: 10.1111/j.1365-313X.2008.03644.x. In press. [DOI] [PubMed] [Google Scholar]
- 50.Cárdenas L, Quinto C. Reactive oxygen species (ROS) as early signals in root hair cells responding to rhizobial nodulation factors. Plant Signal Behav. 2008;3:1–3. doi: 10.4161/psb.3.12.7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 52.Sagi M, Fluhr R. Superoxide production by plant homologues of the gp91(phox) NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol. 2001;126:1281–1290. doi: 10.1104/pp.126.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sagi M, Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006;141:336–340. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, Nara M, Suzuki K, Tanokura M, Kuchitsu K. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem. 2008;283:8885–8892. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell. 2007;19:1065–1080. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, Kojima C, Yoshioka H, Iba K, Kawasaki T, Shimamoto K. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19:4022–4034. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molendijk AJ, Bischoff F, Rajendrakumar CS, Friml J, Braun M, Gilroy S, Palme K. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 2001;20:2779–2788. doi: 10.1093/emboj/20.11.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006;140:1222–1232. doi: 10.1104/pp.105.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SY, Sivaguru M, Stacey G. Extracellular ATP in plants. Visualization, localization and analysis of physiological significance in growth and signaling. Plant Physiol. 2006;142:984–992. doi: 10.1104/pp.106.085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roux SJ, Steinebrunner I. Extracellular ATP: an unexpected role as a signaler in plants. Trends Plant Sci. 2007;12:522–527. doi: 10.1016/j.tplants.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Yang T, Poovaiah BW. Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proc Natl Acad Sci USA. 2002;99:4097–4102. doi: 10.1073/pnas.052564899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]