Abstract
An extract of roots of Centaurea diffusa (diffuse knapweed) yielded caryophyllene oxide and linoleic acid which were shown to be phytotoxic. Also isolated were germacrene B, a previously-known phytotoxin as well as the inactive polyene aplotaxene. A combination of these compounds, if transferred to the soil, could be one factor in the invasive behavior of this weed. Contrary to a literature report, 8-hydroxyquinoline was not detected in root exudates of in vitro grown C. diffusa nor could it be identified in the root extract. However, a recent report from a different group maintains that 8-hydroxyquinoline can be released from roots of C. diffusa following a diurnal rhythm.
Key words: Centaurea diffusa, diffuse knapweed, Asteraceae, caryophyllene oxide, linoleic acid, roots, phytotoxicity
Introduction
Diffuse knapweed, Centaurea diffusa Lam., is an introduced weed which has spread throughout the northwest United States with deleterious effects on range and agricultural areas.1 It has been suggested that plant-plant allelopathy, perhaps caused by phytotoxins exuded or leached from the knapweed, might account for the invasive behavior of this weed. Thus, Muir and Majak2 reported seed germination and seedling growth inhibition by shoot extract fractions and that a sesquiterpene-containing fraction of shoot extracts as well as a polar extract were phytotoxic.3 On the other hand, these researchers could not demonstrate any effects of knapweed litter on plants in range sites and saw no effect on seedlings of other plants grown in the presence of knapweed. Soil extracts from knapweed-infested sites yielded p-coumaric acid, but it only inhibited seed germination and seedling biomass reduction at the highest levels tested. No active compounds were identified from these studies and root exudate extracts yielded only “a series of long-chain saturated and unsaturated carboxylic acids”, which were not tested for phytotoxicity.3 A more recent report4 described the isolation of 8-hydroxyquinoline as a sole phytotoxin from root exudates of in vitro grown C. diffusa, and stated that it was abundant in soil extracts from C. diffusa-invaded North American fields. In contrast, Norton et al.,5 reported that they were unable to detect 8-hydroxyquinoline in experimental or field collected soils from knapweed-infested sites. Roots of C. diffusa have not previously been studied chemically and the novelty of 8-hydroxyquinoline as a plant natural product suggested that analysis of the roots as the likely storage site of this compound might provide useful basic information for a later exploration of possible transport mechanisms for the quinoline into exudates. In conjunction with this, we elected to repeat the exudate analysis and to determine if elicitors could affect the process.
Results and Discussion
Compound isolation and identification.
The crude ethanolic root extract of C. diffusa inhibited the growth of A. thaliana seedlings significantly (Fig. 1). Of the partitioned extract residues, the hexane and CH2Cl2 residues showed some visual phytotoxic effects on A. thaliana seedlings, whereas the n-BuOH fraction did not (data not shown). The hexane fraction exhibited a significantly higher toxicity level than did the CH2Cl2 fraction (Fig. 2), so it was investigated further. Bioassay-driven chromatographic separation of the hexane residue yielded 12 fractions. Fractions 1, 5 and 7 were found to be phytotoxic (data not shown). A GC-MS study on fraction 1 (513 mg) yielded germacrene B (1) (3 mg), (−)-caryophyllene oxide (2) (1 mg), and aplotaxene (3) (15 mg). Analysis of the fraction 5 (305 mg) by GC-MS yielded ethyl linoleate (2 mg), ethyl oleate (3 mg), linoleic acid (4) (20 mg), and (−)-caryophyllene oxide (2) (5 mg) (see Fig. 3). Germacrene B (1) (C15H24) was identified by a GC-MS peak at 11.25 min with mass spectrum (m/z 204, 189, 161, 147, 135 and 12), identical to that of the literature (m/z 204, 189, 161, 147, 136, 121). The structure of caryophyllene oxide (2) (C15H24O) was deduced by a GC-Ms peak at 12.7 min (m/z 220, 87, 177, 161, 149) which was identical to that of the standard commercial sample (m/z 220, 187, 177, 161, 149). In addition, the structures of compounds 3 and 4 were also confirmed by comparison of the NMR spectral data with those reported in the literature.6,7 The 1H NMR spectrum of 3 showed the presence of a terminal vinyl group attached to a methylene group [4.88 (1H, ddd), 4.98 (1H, ddd), 5.78 (1H, ddt)] six olefinic protons [5.30–5.40 (6H, m)], two methylenes α to two double bounds [2.77 (4H, m)], three allylic methylenes [1.98–2.08 (6H, m)] and three adjacent methylenes [1.20–1.40 (6H, m)]. The signal at δ 0.94 (3H, t) was assigned to methyl protons in an ethyl group. These data were identical with those previously reported for aplotaxene.6
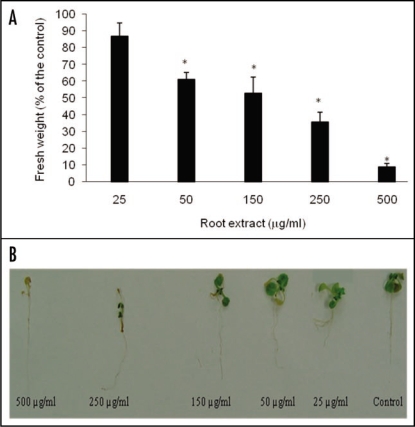
Figure 1.
Growth inhibition of C. diffusa root extract on A. thaliana seedlings. (A) Addition of 50 µg/ml reduced 40% the growth of A. thaliana. Values are presented as percentage differences from the control. Errors bars are the s.e. of the mean, n = 3. *p < 0.05. (B) A. thaliana seedlings treated with 500, 250, 150, 50 and 25 µg/ml C. diffusa root extracts. 50 µl of MeOH was added to control wells.
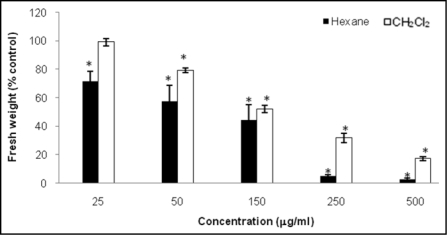
Figure 2.
Phytotoxic activity of hexane and CH2Cl2 root extracts from C. diffusa on A. thaliana seedlings. Values are presented as percentage differences from the control. Errors bars are the s.e. of the mean, n = 4. *p < 0.05.
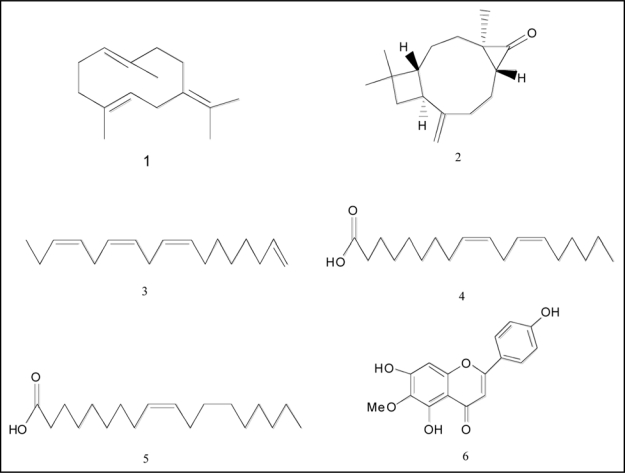
Figure 3.
Compounds present in C. diffusa roots and root exudates.
The 1H NMR spectrum of 4 presented four olefinic protons [5.23–5.40 (4H, m)], a methylene a to two double bounds [2.75 (2H, m)], a methylene adjacent to an ester group [2.25 (2H, m)], two allylic methylenes [1.98 (4H, m)], a methylene [1.66 (2H, m)], seven adjacent methylenes between [(14H, m)] and a terminal methyl group [0.90 (3H, t)]. The spectral properties of this compound were comparable with those reported for linoleic acid.7
The 1H NMR spectrum of fraction 7 (20 mg) showed a complex mixture which was not further investigated.
Fatty acids appear to be chemotaxonomic and biosynthetically less evolved than their counterpart polyacetylenes and are widespread among higher plants. Polyacetylenes are present in a more limited number of family plants, mainly Asteraceae and Apiaceae and commonly occur in a number of Centaurea species,8 but they were not detected in any of the C. diffusa root extracts. Aplotaxene and its derivatives are characteristic compounds of the closely related genera Cirsium and Centaurea.9,10 To what further extent compounds like aplotaxene are of chemotaxonomic importance in these genera is unclear6 and more data are needed.
Germacrene B and related compounds are widespread in Centaurea species.11,12 However, neither germacrene B nor other compounds identified in this study were previously described from C. diffusa.
8-hydroxyquinoline.
The hexane, CH2Cl2, and n-BuOH root extracts were analyzed by LC-MS as described above along with a standard commercial sample of 8-hydroxyquinoline (14.8 min retention time) but none of the quinoline was detected.
Root exudates.
Root exudates elicited with MeJA (12 mg), SA (7 mg), and a non-elicited control (10 mg) were analyzed by LC-MS and NMR. Basically, all showed strong NMR resonances for the presence of a complex mixture of fatty acids with only a trace due to flavonoid-type peaks. The resonances in the crude 1H NMR spectrum in comparison with those data reported in the literature,7 suggested the presence of oleic acid (5) as a major component. Muir et al.3 also reported only long chain fatty acids from their study of C. diffusa root exudates, but these were not further studied.
Definite structures of the other compounds present in the root exudates could not be confirmed due to the small amounts present in comparison to the fatty acid components. Based on EIMS (m/z 300), UV (273.2 nm, 368.3 nm), and the crude 1H NMR spectrum the flavonoid hispidulin (6; 5, 7, 4′-triihydroxy-6-methoxyflavone)13 was identified as a possible component. Hispidulin is a common compound of aerial and root parts of Centaurea species.14–16 Phytotoxic activity of this flavonoid on the germination and growth of crop species is known.17
Contrary to a previous report,4 8-hydroxyquinoline was not detected in the root exudates and these results are complementary to the Norton et al.,5 studies in which it was not found in extracts of C. diffusa soils, either greenhouse-grown or field-collected. However, it should be noted that in a recent study 8-hydroxyquinoline was reported to be secreted by the roots of hydroponically-grown C. diffusa plants following a diurnal pattern.18 In this study the authors reported that 8-hydroxyquinoline was secreted at its maximum by the roots after 6 continuous hours of sun light following a dark period, after 8 hours of sun light the compound started to degrade and after 10 h the compound could not be detected. These studies highlight the transient nature of some root secreted compounds and stress the fact that further studies of 8-hydroxyquinoline are needed to corroborate its presence (or absence) under field conditions. Interestingly, a root secreted compound from Centaurea maculosa, catechin, has been found under field conditions in high concentrations but only at certain times of the year.19
Phytotoxic activity of compounds.
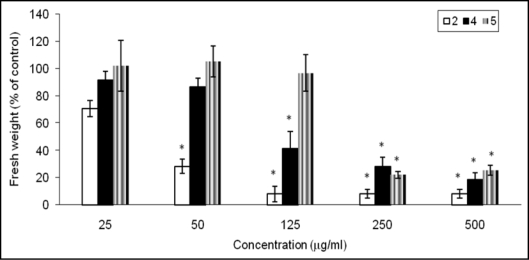
Compounds 2 and 4 were phytotoxic against A. thaliana seedlings (Fig. 4), while aplotaxene (3) was not at the doses tested. Germacrene B (1) has previously been shown to be phytotoxic,20 but we had insufficient material to retest it in this study. The sesquiterpene 2 was the most active compound against A. thaliana seedlings growth. A previous study had shown that fractions containing 2 completely suppressed Schizachyrium scoparium germination but had no effect on lettuce.21 The pure compound itself was not tested. Our results showed that 2 decreased the fresh weight of A. thaliana seedlings by 70% even at 50 µg/ml and caused significant bleaching of the seedlings at phytotoxic concentrations. A bleaching effect has been reported for monoterpene derivatives isolated from another Asteraceae species plant family22 and they were shown to be inhibitors of phytoene desaturase, a key enzyme in carotenoid pigment biosynthesis. Since carotenoids protect chlorophyll from photooxidation, their lack would result in loss of chlorophyll. Some carotenoid biosynthesis inhibitors (Norflurazon and fluridone, for example) are commercial herbicides for control of grasses and aquatic weeds.23 The bleaching of A. thaliana seedlings caused by caryophyllene oxide (2) suggests that it too could be a carotenoid biosynthesis inhibitor.
Figure 4.
Comparison of effect of compounds 2, 4 and 5 found in C. diffusa roots and root exudates on A. thaliana seedling growth. Values are presented as percentage differences from the control. Errors bars are the s.e. of the mean, n = 4. *p < 0.05.
Compound 4 also produced a strong inhibitory effect on A. thaliana, reducing the growth of seedlings by 60% and 80% when the compound was applied in doses between 125 and 500 µg/ml, respectively. Phytotoxicity of this fatty acid on zooplankton24 and germination of some species25 has been previously reported, but its growth inhibitory activity at later stages of plant development, has never been evaluated.
The root-exudate compound 5 decreased growth seedlings by 70% at the two highest concentrations but was inactive at the rest of concentrations tested. The phytotoxicity of 5 has been evaluated in algae24 but this is the first report about its inhibitory growth activity in higher plants.
The higher activity of linoleic acid compared to oleic acid presented in this study is in agreement with the findings of Kakisawa,26 who showed that toxicity of fatty acids increases with an increase in double bonds. Phytotoxic properties of fatty acids are well known and understood. Allelopathic properties of long-chain fatty acids have reported previously on algae by Proctor27 and Kroes.28 Song et al.,29 identified fatty acids among other compounds as putative allelochemicals in rice root exudates.
Further investigations in natural ecosystems where C. diffusa occurs are necessary to confirm the phytotoxic activity observed in vitro against A. thaliana.
The growth stimulating effect of an exudate lipid fraction on ectomycorrhizal fungi in the rhizosphere has been reported before.30 C. diffusa is known to have associations with vesicular arbuscular mycorrhizal fungi (VAM),31 but nothing is known about its ectomycorrhizal fungi associations.
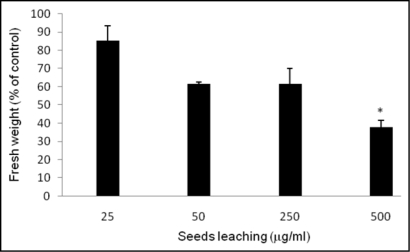
Muir and Majak2 reported that germinating seeds of C. diffusa did not inhibit the seed germination of other species, but suggested that seed chemical production could affect plant growth at later stages. In our study, the C. diffusa seed leachate was evaluated for growth inhibition on A. thaliana (Fig. 5). Significant growth inhibition (p < 0.05) was only observed at the highest dose applied (500 µg/ml). This weak activity is unlikely to have any ecological relevance.
Figure 5.
Effect of seed exudates on A. thaliana growth. Values are presented as percentage differences from the control. Errors bars are the s.e. of the mean, n = 4. *p < 0.05.
The manner in which C. diffusa has became an invasive species in the U.S. is probably complex and not due to any one factor. It possesses some morphological and physiological characteristics that could promote its aggressiveness in the field.1 In addition to its prolific production of viable seeds which germinate under a broad range of environmental conditions, its high tolerance to drought and its monocarpic perennial character should give this species a significant advantage in the ecosystem. As we have discussed, the allelopathic potential of C. diffusa has been a subject of contradictory and controversial studies.2–5,18 Phytotoxic compounds identified in this report could be one of the factors involved in the success of this noxious weed in the colonization of new ecosystems. In addition, lipids present in the root exudates could promote the growth of mycorrhizal fungi in the C. diffusa rhizosphere, which would make it more resistant to soil-borne pathogen attacks.
Materials and Methods
General.
NMR spectra were performed on a Varian INOVA 400 instrument, operating at 400 MHz. The spectra were run in CDCl3; chemical shifts (δ are given in ppm and the coupling constant (J) in hertz (Hz), using TMS as an internal standard.
GC-MS analyses were carried out in an Agilent 5973N GC/MS instrument operated in the electron ionization mode (70 eV) and equipped with a methyl silicone column (30 × 0.25 mm, film thickness 0.25 µm), using He as carrier gas (1 ml min−1), and 2 µl injection size. The injector and MS transfer line temperature were set at 275 and 290°C, respectively. The oven temperature was programmed from 80°C (2 min) to 250°C (5 min) at 10°C min−1. Identification of the different root constituents was performed by a comparison of their retention time and mass spectra with those of standard sample or by mass spectra with those stored in a MS database (Wiley 6N).
For HPLC, a Dionex Co., (Sunnyvale, CA, USA) system was equipped as follows: a P680 pump connected to an ASI-100 automated sampler injector; a PDA-100 photodiode array detector; C18 column (4.6 × 150 mm) with particle size 5 µm; flow rate 0.7 ml min−1; mobile phase 0.1% (v/v) acetic acid in H2O-MeOH; gradient 0 to 10% MeOH 3 min, 10 to 90% MeOH 40 min, 90% MeOH for 15 min.; injection volume 20 µl. The HPLC was coupled to a MSQ-MS detector system (Thermo Electron Co., Waltham, MA, USA), operating in positive mode; electrospray ionization with a N2 flow at 80 psi, a cone voltage of 70 V, needle voltage of 3 kV, and cone temperature 600°C.
Linoleic acid, ethyl linoleate, ethyl oleate, (−)-caryophyllene oxide, 8-hydroxyquinoline, oleic acid, methyl jasmonate and salicylic acid were purchased from Sigma (St. Louis, MO, USA).
Plant material and extract preparation.
Roots of C. diffusa Lam., (2 kg fresh weight) were collected in May 2007 from near Boulder, Colorado, (40° 0′ 46″ N, 105° 17′ 48″ W) USA. The plant was identified by Timothy Seastedt, Prof. of Ecology and Evolutionary Biology, University of Colorado (Boulder, CO, USA).
Freeze-dried roots (526.6 g) were ground into a powder and soaked in 95% EtOH (2 L × 3) at room temp for 3 days. The extract was filtered and evaporated to dryness in vacuo. The concentrated crude extract (16.5 g) was dissolved in MeOH-H2O and partitioned into hexane (5 g), CH2Cl2 (2 g) and n-BuOH (2.5 g) extract residues, successively.
Part of the hexane extract (2 g) was purified on a CombiFlash™ RETRIEVE® (ISCO, Lincoln, NE, USA) system, using a normal phase flash column (RediSep™, ISCO), eluting with hexane and increasing amounts of EtOAc (flow 50 ml/min), to give 12 fractions, which were tested for phytotoxic activity. Active fractions were further analyzed by GC-MS and 1H NMR.
Root exudates.
C. diffusa seeds obtained from the Boulder, Colorado natural populations were soaked overnight at 4°C and surface sterilized in an Eppendorf tube ¾ filled with 50% commercial bleach and 0.1% sodium dodecyl sulfate (SDS) (Shelton scientific, Inc., Shelton, CT, USA). The tube was then vortexed for five min and rinsed five times with sterile ddH2O. Sterilized seeds were germinated on solid Murashige and Skoog (MS) medium (Caisson Laboratories, Inc., Rexburg, ID, USA) supplemented with gibberelic acid (Sigma Chemical Co., St. Louis, MO, USA) in a growth chamber (Percival Scientific, IO, USA) with a 16 h daily light period at 25°C. Ten-day-old seedlings were transferred to previously sterilized 50 ml test tubes (Bellco Biotechnology, Vineland, NJ, USA) with 10 ml of liquid MS medium supplemented with 3% sucrose. Plant cultures were maintained in a rotatory shaker at 90 rpm under cool-white fluorescent lights (45 µmol m−2 s−1) with a 16 h daily light period at 25°C. Thirty-day-old plants were elicited with methyl jasmonate (MeJA) and salicylic acid (SA). Solutions of MeJA and SA were prepared in ethanol and added separately to 20 C. diffusa seedlings per treatment at a final concentration of 250 µM. The root exudates were collected 3 h after the treatment. A non-elicited control of 20 C. diffusa seedlings was also harvested during the same period of time for separate analysis. Elicited and non-elicited root exudates were filtered through Nylon filters of 0.45-µm pore size (Millipore, Durapore® Membrane Filters, Ireland) and extracted with EtOAc (Fisher Co., Fair Lawn, NJ, USA) three times. The resulting EtOAc extracts were concentrated on a rotavapor to yield oily residues which were subjected to NMR and HPLC-MS analyses.
Seed leaching.
C. diffusa seeds were added to 500 µl of ddH2O and kept at 4°C overnight. The resultant liquid was filtered through Nylon filters of 0.45-µm pore size (Millipore, Durapore® Membrane Filters, Ireland) prior to lyophilization (Labconco Kansas City, MO, USA).
Phytotoxic activity assay.
Root extracts, fractions and pure compounds were evaluated for growth inhibition on Arabidopsis thaliana seedlings. A. thaliana seeds (Lehle Seeds, Round Rock, TX, USA) were surface-sterilized with sodium hypoclhorite (3% v/v) for one min, washed five times in distilled sterile ddH2O and germinated on solid MS (Caisson Laboratories, Inc., Rexburg, ID, USA) medium in a growth chamber (Percival Scientific, IO, USA) with a 16 h daily light period at 25°C. The 7-d-old seedlings were transferred into 1 ml of liquid MS medium supplemented with 1% sucrose in 24-well plates (VWR Scientific) and incubated on a rotary shaker at 90 rpm. After 24 h, in four replicate experiments the plants were treated with four concentrations of each test solution (25, 50, 250 and 500 µg ml−1) and a control. Samples to be tested were dissolved in MeOH or DMSO, and applied to the 24-well plates containing the seedlings. Pure MeOH or DMSO (50 µl) was added to control wells. After treatment, plates were sealed with Parafilm® (Pechiney Plastic Packaging, Menasha, WI, USA), incubated on a rotary shaker and grown for seven days under cool-white fluorescent lights (45 µmol m−2 s−1) with a 16 h daily light period at 25°C. After this period the fresh weight of each plant was recorded and the percentage of the fresh weight of the plants was determined by reference to the fresh weight of control plants.
The statistical significance of plant growth inhibition relative to controls between extracts, fractions and pure compounds was determined by a Student's t-test, calculating the mean values and their population variance. The level of significance was p < 0.05.
Acknowledgements
We thank Don Dick for his assistance with GC-MS analysis and Prof. T. Seastedt (University of Colorado) for C. diffusa seeds. The work was supported in part by a grant from U.S. Department of Defense SERDP (SI-1388).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7487
References
- 1.Watson AK, Renney AJ. Biology of Canadian weeds 6. Centaurea diffusa and Centaurea maculosa. Can J Plant Sci. 1974;54:687–701. [Google Scholar]
- 2.Muir AD, Majak W. Allelopathic potential of diffuse knapweed (Centaurea diffusa) extracts. Can J Plant Sci. 1983;63:989–996. [Google Scholar]
- 3.Muir AD, Majak W, Balza F, Towers GHN. A search for the allelopathic agents in diffuse knapweed. Acs Symposium Series. 1987;330:238–246. [Google Scholar]
- 4.Vivanco JM, Bais HP, Stermitz FR, Thelen GC, Callaway RM. Biogeographical variation in community response to root allelochemistry: novel weapons and exotic invasion. Ecol Lett. 2004;7:285–292. [Google Scholar]
- 5.Norton AP, Blair AC, Hardin JG, Nissen SJ, Brunk GR. Herbivory and novel weapons: no evidence for enhanced competitive ability or allelopathy induction of Centaurea diffusa by biological controls. Biological Invasions. 2008;10:79–88. [Google Scholar]
- 6.Christensen LP. Aplotaxene derivatives from Cirsium helenioides. Phytochemistry. 1992;31:2039–2041. [Google Scholar]
- 7.Spectral Database for Organic Compounds. SDBS.
- 8.Champagne DE, Arnason JT, Philogene BJR, Morand P, Lam J. Light-mediated allelochemical effects of naturally-ocurring polyacetylenes and thiophenes from Asteraceae on herbivorous insects. J Chem Ecol. 1986;12:835–858. doi: 10.1007/BF01020255. [DOI] [PubMed] [Google Scholar]
- 9.Christensen LP, Lam J. Acetylenes and related compounds in Cynareae. Phytochemistry. 1990;29:2753–2785. [Google Scholar]
- 10.Christensen LP, Lam J. Flavones and other constituents from Centaurea species. Phytochemistry. 1991;30:2663–2665. [Google Scholar]
- 11.Asadipour AMM, Lari Najafi M. Volatile oil composition of Centaurea aucheri (DC.) Wagenitz. DARU. 2005;13:160–164. [Google Scholar]
- 12.Flamini G, Ertugrul K, Cioni PL, Morelli I, Dural H, Bagci Y. Volatile constituents of two endemic Centaurea species from Turkey: C. pseudoscabiosa subsp pseudoscabiosa and C. hadimensis. Biochem System Eco. 2002;30:953–959. [Google Scholar]
- 13.Gu LWX. Antioxidant activity and components of Salvia plebeian R.Br.—a Chinese herb. Food Chem. 2001;73:299–305. [Google Scholar]
- 14.Trendafilova A, Todorova M, Bancheva S. Secondary metabolites from Centaurea moesiaca. Biochem System Eco. 2007;35:544–548. [Google Scholar]
- 15.Karamenderes C, Bedir E, Pawar R, Baykan S, Khan KA. Elemanolide sesquiterpenes and eudesmane sesquiterpene glycosides from Centaurea hierapolitana. Phytochemistry. 2007;68:609–615. doi: 10.1016/j.phytochem.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Flamini G, Pardini M, Morelli I. A flavonoid sulphate and other compounds from the roots of Centaurea bracteata. Phytochemistry. 2001;58:1229–1233. doi: 10.1016/s0031-9422(01)00345-4. [DOI] [PubMed] [Google Scholar]
- 17.Baruah NC, Sarma JC, Barua NC, Sarma S, Sharma RP. Germination and growth-inhibitory sesquiterpene lactones and a flavone from Tithonia diversifolia. Phytochemistry. 1994;36:29–36. [Google Scholar]
- 18.Tharayil NBP, Alpert P, Walker E, Amarasiriwardena D, Xing B. Dual purpose secondary compounds: Phytotoxins of Centaurea diffusa also facilitate nutrient uptake. New Phytologist. 2008 doi: 10.1111/j.1469-8137.2008.02647. [DOI] [PubMed] [Google Scholar]
- 19.Perry LG, Thelen GC, Ridenour WM, Callaway RM, Paschke MW, Vivanco JM. Concentrations of the allelochemical (+/−)-catechin in Centaurea maculosa soils. J Chem Ecol. 2007;33:2337–2344. doi: 10.1007/s10886-007-9383-1. [DOI] [PubMed] [Google Scholar]
- 20.Kobaisy M, Tellez MR, Dayan FE, Duke SO. Phytotoxicity and volatile constituents from leaves of Callicarpa japonica Thunb. Phytochemistry. 2002;61:37–40. doi: 10.1016/s0031-9422(02)00207-8. [DOI] [PubMed] [Google Scholar]
- 21.Tanrisever N, Fischer NH, Williamson GB. Menthofurans from Calamintha ashei-effects on Schizachyrium scoparium and Lactuca sativa. Phytochemistry. 1988;27:2523–2526. [Google Scholar]
- 22.Perez-Vasquez A, Linares E, Bye R, Cerda-Garcia-Rojas CM, Mata R. Phytotoxic activity and conformational analysis of thymol analogs from Hofmeisteria schaffneri. Phytochemistry. 2008;69:1339–1347. doi: 10.1016/j.phytochem.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Arias RS, Netherland MD, Scheffler BE, Puri A, Dayan FE. Hydrilia verticillata and its potential use to generate herbicide-resistant crops. John Wiley & Sons Ltd: 2005. Molecular evolution of herbicide resistance to phytoene desaturase inhibitors; pp. 258–268. [DOI] [PubMed] [Google Scholar]
- 24.Chiang IZ, Huang WY, Wu JT. Allelochemicals of Botryococcus braunii (Chlorophyceae) J Phycol. 2004;40:474–480. [Google Scholar]
- 25.Edney NA, Rizvi M. Phytotoxicity of fatty acids present in dairy and hog manure. J Environ Sci and Heal B. 1996;31:269–281. [Google Scholar]
- 26.Kakisawa H, Asari F, Kusumi T, Toma T, Sakurai T, Oohusa T, Hara Y, Chihara M. An allelopathic fatty-acid from the brown alga Cladosiphon okamuranus. Phytochemistry. 1988;27:731–735. [Google Scholar]
- 27.Proctor VW. Studies of algal antibiosis using Haematococcus and Chlamydomonas. Limnol Oceanogr. 1957;2:125–139. [Google Scholar]
- 28.Kroes HW. Growth interactions between Chlamydomonas globosa snow and Chlorococcum ellipsoideum deason and bold—role of extracellular products. Limnol Oceanogr. 1972;17:423. doi: 10.1007/BF00425205. [DOI] [PubMed] [Google Scholar]
- 29.Song HK, Ahn JK, Ahmad A, Hahn SJ, Kim SH, Chung IM. Identification of allelochemicals in rice root exudates at various phenological phases and their influence on barnyardgrass. Allelopathy J. 2004;13:173–188. [Google Scholar]
- 30.Fries NBM, Serck-Hanssen K. Growth of ectomycorrhizal fungi stimulated by lipids from a pine root exudates. Plant Soil. 1985;86:287–290. [Google Scholar]
- 31.Harris P, Clapperton MJ. An exploratory study on the influence of vesicular-arbuscular mycorrhizal fungi on the success of weed biological control with insects. Biocontrol Sci Techn. 1997;7:193–201. [Google Scholar]