Abstract
Aerobic metabolism inevitably produces reactive oxygen species (ROS), including hydrogen peroxide, which may cause damage to the cell. Besides this toxic effect, hydrogen peroxide has an important signaling function in plant development and response to environmental stimuli. So, the balance of toxic and signaling effects of hydrogen peroxide is highly dependent on mechanisms to adjust its level in the different cell compartments. We recently described a redox system, formed by NADPH thioredoxin reductase (NTR) and 1-Cys peroxiredoxin (1-Cys Prx), able to use the reducing power of NADPH to reduce hydrogen peroxide. This system is localized in the nucleus of wheat seed cells and probably has an important antioxidant function in aleurone and scutellum cells, which suffer oxidative stress during seed development and germination. We discuss here the possibility that the control of the level of hydrogen peroxide in the nucleus may be important to balance redox regulation of gene expression and cell death in cereal seed cells.
Key words: thioredoxin reductase, peroxiredoxin, hydrogen peroxide, signaling, cell death, oxidative stress, cereal seed
Introduction
Life in aerobiosis is challenged by the production of reactive oxygen species (ROS) including hydrogen peroxide. In plants, different environmental stimuli increase ROS production; however, there are tissues that naturally suffer oxidative stress during plant growth and development. This is the case of the cereal seed that suffers oxidative stress provoked by the massive loss of water at late stages of development, and after resumption of respiration following germination.1–4 Though during seed development different tissues undergo programmed cell death (PCD),5–7 aleurone and scutellum cells play an essential role in germination and therefore have to survive this oxidative stress. Germination of cereal seeds is activated by gibberellins which induce in aleurone cells the expression of genes encoding hydrolytic enzymes that allow the mobilization of the storage components of the starchy endosperm.8 Once this process is completed, gibberellins activate aleurone PCD,9 which progression takes place after cytoplasmic ROS detoxification systems are downregulated.10 We have recently described that NADPH thioredoxin reductase (NTR) supports the antioxidant activity of 1-Cys peroxiredoxin (1-Cys Prx) using NADPH as source of reducing power. Interestingly, this novel redox system accumulates in the nucleus of seed cells suffering oxidative stress.11
Signaling versus Toxic Effect of Hydrogen Peroxide in the Nucleus of Wheat Seed Cells
The finding that the NTR/1-Cys Prx system accumulates in the nucleus of seed cells suffering oxidative stress, and in vitro assays showing that this system is able to use NADPH to reduce hydrogen peroxide provide evidence of a mechanism to control the oxidant environment of the nucleus. A primary function of this system may be to avoid damage to DNA and nuclear structures, which is probably important taking into account the recent demonstration of ROS production in nuclei of plant cells.12 DNA protection assays in vitro support this detoxification role of the nuclear-localized hydrogen peroxide scavenging system.13,14
However, the control of the level of hydrogen peroxide in the nucleus probably has a signaling function which may involve redox regulation of transcription, as shown in yeast and animal cells.15 In S. pombe the expression of genes of the antioxidant response is regulated by a bZIP transcription factor, Pap1, activated by the formation of an internal disulfide bridge upon H2O2 treatment.16 Although the available information on redox regulation of gene expression in plants is still scarce,17 the DNA binding activity of several transcription factors depends on the redox environment.18–20 In the context of gene expression in cereal seeds, it was shown that P1, a R2R3-type MYB transcription factor from maize, requires reducing conditions for DNA binding.21,22 This finding is interesting because a masterpiece of gene regulation in response to gibberellins in aleurone cells from cereal seeds, GAMYB,23 is a R2R3-type MYB transcription factor. Though no redox regulation of its activity has been reported so far, an Arabidopsis line overexpressing 1-Cys Prx showed lower germination efficiency than wild type,14 suggesting that the nuclear redox environment plays an important role in the activation of germination.
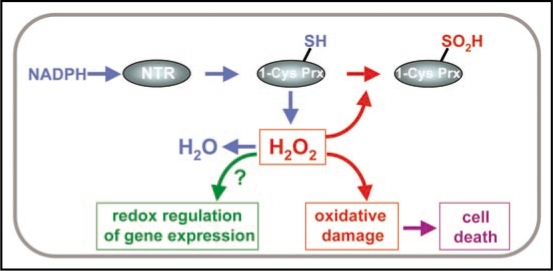
A remarkable feature of the nuclear NTR/1-Cys Prx system is the sensitivity of the 1-Cys Prx to oxidant conditions, which provoke overoxidation of the peroxidatic Cys residue to sulfinic acid, thus inactivating the enzyme.11 Inactivation by overoxidation is a well-described characteristic of eukaryotic 2-Cys Prxs,24 important for hydrogen peroxide-dependent signaling in eukaryotes.25,26 Whilst the overoxidation of 2-Cys Prx to sulfinic acid is reversible,27 overoxidation of 1-Cys Prx seems to be irreversible.28 Therefore, the progressive overoxidation of the nuclear 1-Cys Prx will probably increase the oxidant environment of the nucleus, according to the scheme depicted in Figure 1. The level of hydrogen peroxide in the nucleus may influence gene expression as shown for the redox regulation of the yeast Pap1 transcription factor, which is mediated by a 2-Cys Prx at low concentration of H2O229,30 but not at high concentration of H2O2 because the 2-Cys Prx becomes overoxidized.30 Therefore, in germinating seeds the nuclear NTR/1-Cys Prx redox system would control the level of hydrogen peroxide allowing gene expression. Then, as 1-Cys Prx is progressively inactivated by overoxidation, the nuclear environment is likely to become more oxidant, thus favouring cell death9 (Fig. 1). In yeast and animal cells hydrogen peroxide promotes DNA cleavage mediated by toposimerase I and II.31,32 Whether the increase of hydrogen peroxide provokes cell death in cereal seeds through the damage of nuclear structures or by the regulation of signal transduction events, as occurs in animal cells,33 is not yet known.
Figure 1.
The nuclear-localized redox system formed by NTR and 1-Cys Prx is able to use NADPH to detoxify hydrogen peroxide in cereal seeds suffering oxidative stress. This system may control the oxidant conditions in the nucleus, which is probably important for redox regulation of gene expression in germinating seed cells. As 1-Cys Prx is progressively inactivated by overoxidation, the increase of the oxidant conditions in the nucleus promote oxidative damage and cell death.
Acknowledgements
This work was supported by Grant BIO2007-60644 from Ministerio de Educación y Ciencia, Spain, and Grants CVI-182 and P06-CVI-01578 from Junta de Andalucía, Spain. P.P. was recipient of a pre-doctoral fellowship from Universidad de Sevilla.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7221
References
- 1.Leprince O, Hendry GAF, McKersie BD. The mechanisms of desiccation tolerance in developing seeds. Seed Sci Res. 1993;3:231–246. [Google Scholar]
- 2.Leprince O, Atherton NM, Deltour R, Hendry GAF. The involvement of respiration in free radical processes during loss of desiccation tolerance in germinating Zea mays L. Plant Physiol. 1994;104:1333–1339. doi: 10.1104/pp.104.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serrato AJ, Crespo JL, Florencio FJ, Cejudo FJ. Characterization of two thioredoxins hwith predominant localization in the nucleus of aleurone and scutellum cells of germinating wheat seeds. Plant Mol Biol. 2001;46:361–371. doi: 10.1023/a:1010697331184. [DOI] [PubMed] [Google Scholar]
- 4.Serrato AJ, Cejudo FJ. Type-h thioredoxins accumulate in the nucleus of developing wheat seed tissues suffering oxidative stress. Planta. 2003;217:392–399. doi: 10.1007/s00425-003-1009-4. [DOI] [PubMed] [Google Scholar]
- 5.Domínguez F, Cejudo FJ. Germination-related genes encoding proteolytic enzymes are expressed in the nucellus of developing wheat grains. Plant J. 1998;15:569–574. [Google Scholar]
- 6.Domínguez F, Moreno J, Cejudo FJ. The nucellus degenerates by a process of programmed cell death during the early stages of wheat grain development. Planta. 2001;213:352–360. doi: 10.1007/s004250000517. [DOI] [PubMed] [Google Scholar]
- 7.Young TE, Gallie DR. Regulation of programmed cell death in maize endosperm by abscisic acid. Plant Mol Biol. 2000;42:397–414. doi: 10.1023/a:1006333103342. [DOI] [PubMed] [Google Scholar]
- 8.Domínguez F, Cejudo FJ. Patterns of starchy endosperm acidification and protease gene expression in wheat grains following germination. Plant Physiol. 1999;119:81–88. doi: 10.1104/pp.119.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domínguez F, Moreno J, Cejudo FJ. A gibberellin-induced nuclease is localized in the nucleus of wheat aleurone cells undergoing programmed cell death. J Biol Chem. 2004;279:11530–11536. doi: 10.1074/jbc.M308082200. [DOI] [PubMed] [Google Scholar]
- 10.Fath A, Bethke PC, Jones RL. Enzymes that scavenge reactive oxygen species are downregulated prior to gibberellic acid-induced programmed cell death in barley aleurone. Plant Physiol. 2001;126:156–166. doi: 10.1104/pp.126.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulido P, Cazalis R, Cejudo FJ. An antioxidant redox system in the nucleus of wheat seed cells suffering oxidative stress. Plant J. 2008 doi: 10.1111/j.1365-313X.2008.03675.x. In press. [DOI] [PubMed] [Google Scholar]
- 12.Ashtamker C, Kiss V, Sagi M, Davydov O, Fluhr R. Diverse subcellular locations of cryptogein-induced reactive oxygen species production in tobacco bright yellow-2 cells. Plant Physiol. 2007;143:1817–1826. doi: 10.1104/pp.106.090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stacy RAP, Munthe E, Steinum T, Sharma B, Aalen R. A peroxiredoxin antioxidant is encoded by a dormancy-related gene, Per1, expressed during late development in the aleurone and embryo of barley grains. Plant Mol Biol. 1996;31:1205–1216. doi: 10.1007/BF00040837. [DOI] [PubMed] [Google Scholar]
- 14.Haleskas C, Viken MK, Grini PE, Nygaard V, Nordgard SH, Meza TJ, Aalen RB. Seed 1-Cysteine peroxiredoxin antioxidants are not involved in dormancy, but contribute to inhibition of germination during seed stress. Plant Physiol. 2003;133:1148–1157. doi: 10.1104/pp.103.025916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Toone WM, Kuge S, Samuels M, Morgan BA, Toda T, Jones N. Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (Exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev. 1998;12:1453–1463. doi: 10.1101/gad.12.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietz K-J. Redox signal integration: from stimulus to networks and genes. Physiol Plant. 2008;133:459–468. doi: 10.1111/j.1399-3054.2008.01120.x. [DOI] [PubMed] [Google Scholar]
- 18.Comelli RN, González DH. Conserved homeodomain cysteines confer redox sensitivity and influence the DNA binding properties of plant class IIIHD-Zip proteins. Arch Biochem Biophys. 2007;467:41–47. doi: 10.1016/j.abb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Rochon A, Boyle P, Wignes T, Fobert PR, Despres C. The coactivator function of Arabidopsis NPR1 requires the core of its BTB/POZ domain and the oxidation of C-terminal cysteines. Plant Cell. 2006;18:3670–3685. doi: 10.1105/tpc.106.046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaikhali J, Heiber I, Seidel T, Ströer E, Hiltscher H, Birkmann S, Dietz KJ, Baier M. The transcription factor Rap2.4a confers redox sensitivity to nuclear expression of chloroplast antioxidant enzymes. BMC Plant Biol. 2008;8:48. doi: 10.1186/1471-2229-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams CE, Grotewold E. Differences between plant and animal Myb domains are fundamental for DNA binding activity, and chimeric Myb domains have novel DNA binding specificities. J Biol Chem. 1997;272:563–571. doi: 10.1074/jbc.272.1.563. [DOI] [PubMed] [Google Scholar]
- 22.Heine GF, Hernández JM, Grotewold E. Two cysteines in plant R2R3 MYB domains participate in redox-dependent DNA binding. J Biol Chem. 2004;279:37878–37885. doi: 10.1074/jbc.M405166200. [DOI] [PubMed] [Google Scholar]
- 23.Gubler F, Kalla R, Roberts JK, Jacobsen JV. Gibberellin-regulated expression of a myb gene in barley aleurone cells: evidence for Myb transactivation of a high-pI alpha-amylase gene promoter. Plant Cell. 1995;11:1879–1891. doi: 10.1105/tpc.7.11.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signalling. Free Rad Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signalling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 26.Georgiou G, Masip L. An overoxidation journey with a return ticket. Science. 2003;300:592–594. doi: 10.1126/science.1084976. [DOI] [PubMed] [Google Scholar]
- 27.Biteau B, Labarre J, Toledano M. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 28.Woo HA, Jeong W, Chang T-S, Par KJ, Park SJ, Yang JS, Rhee SG. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-Cys peroxiredoxins. J Biol Chem. 2005;280:3125–3128. doi: 10.1074/jbc.C400496200. [DOI] [PubMed] [Google Scholar]
- 29.Bozonet SM, Findlay VJ, Day AM, Cameron J, Veal EA, Morgan BA. Oxidation of eukaryotic 2-Cys peroxiredoxin is a molecular switch controlling the transcriptional response to increasing levels of hydrogen peroxide. J Biol Chem. 2005;280:23319–23327. doi: 10.1074/jbc.M502757200. [DOI] [PubMed] [Google Scholar]
- 30.Vivancos AP, Castillo EA, Biteau B, Nicot C, Ayté J, Toledano MB, Hidalgo E. A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc Natl Acad Sci USA. 2005;102:8875–8880. doi: 10.1073/pnas.0503251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li T-K, Chen AY, Yu C, Mao Y, Wang H, Liu LF. Activation of topoisomerase II-mediated excision of chromosomal DNA loops during oxidative stress. Genes Dev. 1999;13:1553–1560. doi: 10.1101/gad.13.12.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daroui P, Desai SD, Li T-K, Liu AA, Liu LF. Hydrogen peroxide induces topoisomerase I-mediated DNA damage and cell death. J Biol Chem. 2004;279:14587–14594. doi: 10.1074/jbc.M311370200. [DOI] [PubMed] [Google Scholar]
- 33.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nature Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]