Abstract
Oligogalacturonides (OGs) are endogenous elicitors of defense responses released after partial degradation of pectin in the plant cell wall. Despite OGs cannot be considered true pathogen-associated molecular patterns, such as Flg22, they can be considered host-associated molecular patterns that are generated by the host cell during the infection process, and that stimulate the plant innate immune system. We have previously shown that, in Arabidopsis, OGs increase resistance to Botrytis cinerea independently of jasmonate, salicylic acid and ethylene. Recently, we demonstrated that, in Arabidopsis, OGs elicit a robust extracellular oxidative burst that is generated through the NADPH-oxidase AtrbohD. Moreover, we showed that this burst is dispensable either for early expression of OG-induced marker genes or for OG-induced resistance to B. cinerea. Similarly to Flg22, stimulation with OGs leads to the phosphorylation of mitogen activated protein kinase 3 and 6, suggesting that, even though different elicitors are perceived by distinct receptors, the signalling pathways mediated by these molecules converge very early and lead to the stimulation of the innate immune system.
Key words: oligogalacturonides, oxidative burst, Botrytis cinerea, elicitors, plant-pathogen interactions
Because of their sessile lifestyle, plants cannot run away from invaders and need to defend themselves from threatening organisms by mounting a wide array of defense responses in a timely manner. Due to the absence of an adaptive immune system, plants rely on a so-called “innate immune system”, analogous to that found in animals.1,2 Upon perception of pathogen-derived molecules, collectively called elicitors, plants activate inducible defense responses, including the production of reactive oxygen species (ROS) and the accumulation of antimicrobial compounds (phytoalexins) and pathogenesis-related proteins.2
Many elicitors are secreted or are present on the surface of most strains of a given microbial taxonomic group and can activate defense responses effective against a wide range of pathogens. For this reason, they are also referred to as pathogen-associated molecular patterns (PAMPs).3,4 PAMPs are often structural components of the pathogen cell wall (e.g., chitin, glucan) or other macromolecular structures (e.g., bacterial flagellin).5 A prototypical PAMP is Flg22 (a 22 amino acid peptide derived from the N-amino terminal region of flagellin) which is able to induce defense responses in different species such as tomato and Arabidopsis thaliana. Pathogen-secreted cell wall-degrading enzymes (for example polygalacturonases, PGs) also act as elicitors. Many PGs are not elicitors per se, but are rather able to release elicitor-active molecules from the host cell wall. When the activity of a fungal PG is modulated by apoplastic PG-inhibiting proteins (PGIPs), long-chain oligogalacturonides (OGs) are produced.6,7 OGs cannot be considered true PAMPs, since they are not derived from the pathogen. However, they can be considered host-associated molecular patterns (HAMPs) that are generated by the host cell during the infection process.
To date, the molecular basis underlying the recognition of OGs and the subsequent activation of plant defense responses is not well understood. We have recently shown that, in Arabidopsis, OGs can activate defense responses effective against the fungal pathogen Botrytis cinerea and that OG-induced protection is independent of salicylic acid, ethylene and jasmonic acid,8 similarly to Flg22-induced resistance to Pseudomonas syringae.9 Furthermore, genome-wide transcript profile analysis of Arabidopsis seedlings treated with either OGs or Flg22 for 1 hour indicates an extensive overlap of transcriptional changes triggered by these elicitors.10 However, the changes induced by Flg22 are more conspicuous, both in terms of fold-change and number of genes affected, and more sustained (indeed, after 3 hours of treatment most changes induced by OGs at 1 hour drop to basal levels). Transcriptional data suggest that OGs and Flg22 may stimulate the plant innate immune system through the activation of shared signalling pathways. To investigate this hypothesis, we have performed a detailed analysis of early and late responses induced by OGs in Arabidopsis. In particular, we focused our attention on the oxidative burst, because it is a hallmark of early recognition of elicitors and pathogens,11 likely playing an important role in the activation of downstream responses. Our data indicate that the source of the oxidative burst induced by OGs is the NADPH oxidase AtrbohD,12 which was previously shown to catalyze the production of ROS in response to pathogens13 and Flg22.14 The oxidative burst is not required for early expression of OG-induced marker genes (PAD3, RetOx, AtPGIP1, WRKY40 and CYP81F2), but it is necessary for full accumulation of callose,12 consistently with what observed in Flg22-treated plants.15 Furthermore, AtrbohD is dispensable for OG-induced resistance to B. cinerea,12 in stark contrast with Flg22-induced protection against P. syringae.15
It is well known that Mitogen-Activated Protein Kinases (MAPKs) are promptly activated upon elicitor perception.16 In particular, in Arabidopsis, a MAPK cascade, leading to AtMPK3 and AtMPK6 activation, is required for Flg22-mediated responses.17 We have also observed that OGs rapidly activate AtMPK310 and AtMPK6 (data not shown), suggesting that, even though OGs and Flg22 are perceived by distinct receptors,10 the signalling pathways mediated by these elicitors converge very early.
In conclusion, our results and data recently reported by other researchers indicate that PAMPs and HAMPs are able to activate plant innate immunity using a common signalling pathway which involves a MAPK cascade (Fig. 1). However, this pathway bifurcates into at least two branches: activation of one branch requires the oxidative burst mediated by AtrbohD and is important against bacterial pathogens, whereas the oxidative burst-independent branch regulates defense responses effective against necrotrophic fungi. So, PAMPs and HAMPs, despite their distinct origin, are functionally equivalent in many aspects. Similarly, in mammalian cells, the breakdown of hyaluronan, a component of the extracellular matrix, activate the innate immune system. Indeed, the perception of hyaluronan fragments through the leucine-rich repeat receptor kinases TLR2 and TLR4, which are also required for the perception of PAMPs, leads to the activation of inflammatory responses.18 It is therefore clear that both plants and animals have evolved a common system to activate the innate immunity in response to both pathogen- and host-derived signals of danger.
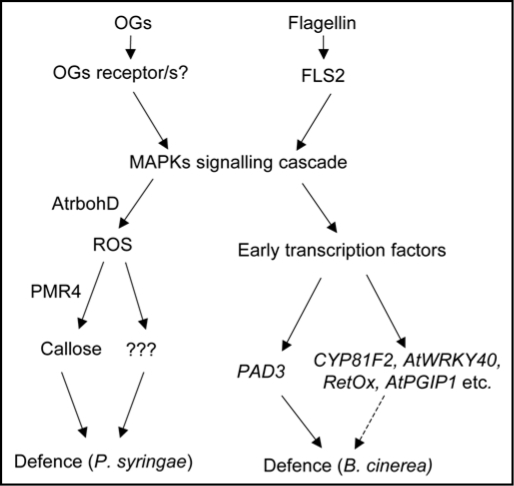
Figure 1.
Model for HAMP- and PAMP-induced signalling pathway. The perception of OGs by their receptor/s or Flg22 by FLS2 activates AtMPK3 and AtMPK6 that subsequently trigger gene expression and AtrbohD-mediated ROS production, callose accumulation and other defence responses. Responses activated independently of ROS (such as PAD3 expression) are required for resistance against Botrytis cinerea, whereas ROS-dependent responses restrict bacterial growth.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7224
References
- 1.Gómez-Gómez L. Plant perception systems for pathogen recognition and defence. Mol Immunol. 2004;41:1055–1062. doi: 10.1016/j.molimm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Nurnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- 3.Parker JE. Plant recognition of microbial patterns. Trends Plant Sci. 2003;8:245–247. doi: 10.1016/S1360-1385(03)00105-5. [DOI] [PubMed] [Google Scholar]
- 4.He P, Shan L, Sheen J. Elicitation and suppression of microbe-associated molecular pattern-triggered immunity in plant-microbe interactions. Cell Microbiol. 2007;9:1385–1396. doi: 10.1111/j.1462-5822.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 5.Nürnberger T, Brunner F. Innate immunity in plants and animals: emerging parallels between the recognition of general elicitors and pathogen-associated molecular patterns. Curr Opin Plant Biol. 2002;5:318–324. doi: 10.1016/s1369-5266(02)00265-0. [DOI] [PubMed] [Google Scholar]
- 6.De Lorenzo G, D'Ovidio R, Cervone F. The role of polygacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu Rev Phytopathol. 2001;39:313–335. doi: 10.1146/annurev.phyto.39.1.313. [DOI] [PubMed] [Google Scholar]
- 7.De Lorenzo G, Ferrari S. Polygalacturonase-inhibiting proteins in defense against phytopathogenic fungi. Curr Opin Plant Biol. 2002;5:295–299. doi: 10.1016/s1369-5266(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J. Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene or jasmonate signaling but requires PAD3. Plant Physiol. 2007;144:367–379. doi: 10.1104/pp.107.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 10.Denoux C, Galletti R, Mammarella N, Gopalan S, Werck G, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J. Activation of defense response pathways by OGs and flg22 elicitors in Arabidopsis Seedlings. Mol Plant. 2008;1:423–445. doi: 10.1093/mp/ssn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 12.Galletti R, Denoux C, Gambetta S, Dewdney J, Ausubel FM, De Lorenzo G, Ferrari S. The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis thaliana is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Plant Physiol. 2008;148:1695–1706. doi: 10.1104/pp.108.127845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres MA, Dangl JL, Jones JD. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuhse TS, Bottrill AR, Jones AM, Peck SC. Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J. 2007;51:931–940. doi: 10.1111/j.1365-313X.2007.03192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, Chen S, Tang X, Zhou JM. A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microb. 2007;1:175–185. doi: 10.1016/j.chom.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Pedley KF, Martin GB. Role of mitogen-activated protein kinases in plant immunity. Curr Opin Plant Biol. 2005;8:541–547. doi: 10.1016/j.pbi.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 18.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]