Abstract
Plant viruses spread cell-to-cell in infected plants by exploiting plasmodesmata (PD), gatable channels in the cell wall that provide cytoplasmic passageways for the trafficking of informational macromolecules. Since it became known that the intercellular spread of Tobacco mosaic virus (TMV) depends on virus-encoded movement protein (MP), the mechanism by which this protein mediates in the targeting of this virus to PD is subject to intense studies. TMV movement occurs in a non-encapsidated form and thus promises to reveal important host functions involved in the intra-and intercellular trafficking of RNA molecules. We have recently presented new evidence that the cell-to-cell trafficking of TMV RNA (vRNA) involves the formation and intracellular trafficking of distinct MP particles. Upon assembly, these particles detach from cortical microtubule (MT) sites and then move with the flow of ER through the cell. During passage the particles continue to undergo transient interactions with MT which may guide the particles to their destination. The comprehensive analysis of particle composition may lead to important insights into the regulation of RNA transport in plants and may also reveal potential similarities to RNA transport mechanisms in animals and humans.
Key words: Tobacco mosaic virus, movement protein, RNA transport, plasmodesmata, microtubules, endoplasmic reticulum
To support the spread of TMV infection,1 the MP accumulates in PD2 and modifies their size exclusion limit.3 The protein also binds single-stranded nucleic acids in vitro,4 and thus may form a complex with vRNA to facilitate vRNA transport from sites of replication to PD. Consistent with the notion that most if not all plant RNA viruses replicate in association with membranes, the MP has predicted transmembrane domains5 and cofractionates with membranes isolated from infected cells.6 The use of TMV derivatives expressing functional MP:GFP fusion protein led to the visualization of cellular MP-interacting components that may have a role in the targeting of the MP and/or the vRNP to PD.7–9 Initial observations concentrated on cells behind the leading front of infection where high levels of fluorescent MP:GFP accumulate.8–10 In such cells, the protein occurs in association with ER membrane-containing inclusion bodies (IB), which contain viral replicase and vRNA,8,11 and thus likely represent aggregates of ER and viral replication complexes (VRCs). Consistent with the association of the ER with actin,12 these ER aggregates occur in proximity to actin microfilaments (MF), which contribute to their formation, their size and their mobility.13,14 Given that ER membranes are continuous between cells through PD,15,16 they provide a potential pathway for the movement of membrane-associated VRCs from the infected cells into adjacent cells. This model of TMV movement appears possible since ER-targeted membrane proteins are able to laterally diffuse within the membrane17,18 and to move cell-to-cell.17 Moreover, conditions that affect the structure of the ER or of the associated MF network can reduce the efficiency by which the MP is targeted to PD.19
In addition to IB, the MP also accumulates in association with MT7,8 and functional studies involving various MP mutations, including conditional mutations, have demonstrated that the ability of MP to interact with MT tightly correlates with the vRNA movement function of the protein in leading front cells.20 However, because of the very low amount of MP:GFP in leading front cells, the exact function of MT in the vRNA movement process has been elusive. The role of MT and MF in TMV movement was addressed by indirect approaches using chemical drugs.10,13,14,21 However, although these drugs may cause specific defects in the TMV transport pathway, these defects are difficult to detect at the level of TMV spread, since the spread of infection requires only low amounts of MP22 and only few spreading viral genomes, and thus occurs unaltered if the transport pathway is not completely blocked.23 A measurable effect on TMV spread was observed upon disruption of the actin cytsokeleton for several days.13,14 However, because of the duration of actin poisoning it seems possible that unspecific secondary effects might play a role. The application of actin polymerization inhibitors led to contrasting observations with respect to the effects on the targeting of MP to PD.19,24
Novel insights into the cellular events with relevance to the mechanism supporting the intercellular spread of vRNA were achieved when leading front cells of infection sites in leaves caused by a TMV derivative encoding a temperature-sensitive MP fused to GFP were analysed by highly sensitive digital video microscopy.25 These studies revealed the occurrence of small MP particles emanating from IB at permissive temperature. Similar particles were then also observed in leading front cells of infection sites produced by a virus encoding wild type MP. Moreover, the analysis of infection sites in plants expressing GFP-tagged α-tubulin revealed that the mobile particles occur proximal to MT.
Our recent publication26 describes transient expression experiments to further characterize the particle movements and their role in vRNA trafficking. The MP particles produced under transient expression conditions are functionally significant and functionally related to the particles in infected cells because in either system the formation of the particles is temperature-sensitive in the presence of a temperature-sensitive mutation in MP, which also confers temperature-sensitivity to the intercellular vRNA movement function of the protein. Using a MP fused to RFP and by using GFP to label the mRNA which encodes MP:RFP, evidence is demonstrated that the MP mediates the targeting of its own mRNA to PD. Moreover, these experiments provide first indications that GFP-tagged mRNA occurs in mobile MP:RFP particles, thus suggesting that MP binds RNA in vivo and mediates RNA transport through the formation of an RNP complex. Mobile RNA particles were also observed upon the labeling of the mRNA of RFP, thus in the absence of MP. However, RFP mRNA did not accumulate in PD, thus indicating that the accumulation of RNA in PD is a function of MP. The observation of RFP mRNA particles may suggest that MP associates with an existing RNA transport mechanism and functions by deviating this transport pathway towards PD.
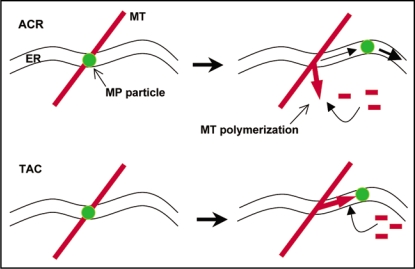
Moreover, using plants in which either the ER or the MT are labeled with GFP the movements of the particles are shown to occur with the flow of ER. Interestingly, in advance and during the movement with the ER the particles transiently anchor to MT proximal sites. When the cells are treated with MT disrupting agent and observed over time, the movements of the particles are halted before MT depolymerize, thus suggesting that the particle movements require dynamic MT. The interactions of the particles with MT during early infection may represent the mechanism that seeds the accumulation of MP along the MT during later stages of infection, thus after MP has completed its vRNA movement function. The MP may interact with MT through direct binding as is suggested by the ability of MP to bind tubulin and MT in vitro and also to bind in vivo to MT in various plant and non-plant cell systems.21,27 Consistent with the observations suggesting interactions of MP with dynamic MT during early infection stages, the MP has the capacity to interact with microtubule end-binding protein 1 (EB1)28 as well as with γ-tubulin,26 thus with key players in the regulation of MT polymerization dynamics. Based on these findings, we propose that TMV movement involves the transport of MP and of MP-vRNA particles with the flow of the ER membrane. MT serve as sites for the assembly of movement-compentent RNPs and may provide positional information that guides the RNP particles to PD. As shown in Figure 1, we propose an “attachment complex release” (ACR) mechanism, which involves MT polymerization to mediate the controlled release of the assembled particle from MT proximal sites. As the particle moves with the ER and encounters a new MT, the particle docks again and may be quality-checked before being released again. Potentially, MT may also provide motive force to the ER-mediated transport of the RNP particles. Thus, in an alternative model, a polymerizing MT end attaches to the ER-associated RNP particle through interaction with MP and pushes the particle into a given direction. Although MP particles particularly localizing to the tips of growing MTs were not observed, such “tip-attachment complex” (TAC) mechanism has been described for the extension of ER tubules in mammalian cells.29
Figure 1.
Hypothetical ACR and TAC mechanisms by which dynamic microtubules could participate in the controlled maturation and trafficking of MP-vRNA particles in the ER membrane. ACR, attachment complex release mechanism; TAC, tip attachment complex mechanism.
Following this first characterization of MP particles associated with the transport of RNA to PD, a number of additional questions remain. For example, since the MP binds single-stranded nucleic acids in a sequence-independent manner4 and since MP was recently shown to facilitate the spread of RNA-based silencing signal molecules,30 it will be interesting to determine whether the MP particles can transport different RNA sequences and whether the specific RNA molecules contained in the particles are non-cell-autonomous.
Moreover, although RNA molecules accumulate in PD together with MP, further studies will be needed to reveal whether the particles observed in the cytoplasm indeed represent the vehicles that target RNA to PD or whether they could represent recycling MP-containing vesicles that only form after MP has performed its RNA transport function. Since cytoplasmic macromolecular RNA structures can be of different nature,31 it will important to determine if the observed mobile RNA particles indeed represent transport particles. Given published evidence that TMV movement occurs in the form of intact replication complexes,14 further studies should also be aimed at elucidating whether the MP particles could represent specialized ER subdomains or lipid rafts that translocate in the ER membrane and harbor VRCs. These subdomains may be movement-competent during early stages of infection and may transform into viral factories that eventually grow into IB during later stages. The latter hypothesis may be supported by the observation that several MP particles joined larger MP-containing structures in time-lapse movies.26
Future research should also investigate the extent by which the MP particles may share features with RNA transport particles described in animal systems. Consistent with our observations, RNA transport in animal systems usually involves MT32–35 and in some cases also the ER,36,37 and it will be interesting to determine whether conserved mechanisms play a role. Studies in neurons have shown that RNA molecules within transport particles are translationally repressed and that the particles contain, in addition to several RNA-binding proteins, important components of the translation machinery that may be needed to initiate translation when the particles reach their final destination.32 Intriguingly, there is evidence indicating that also the RNA of TMV is translationally repressed during transport. Thus, translation of TMV RNA appears to occur only after transport into recipient cells and is controlled by a process involving phosphorylation of MP by protein kinase C.38,39 Should the RNA carried in MP particles indeed be translationally repressed, the next exciting question might be whether this repression involves sRNAs. Recent findings indicate that translational repression by miRNAs in plants involves MT dynamics.40 This suggests the exciting possibility that dynamic MTs, from which MP particles are released upon maturation for transport with the ER, represent cellular sites at which the transport particles are formed and at which translational repression of RNA is established. In this respect in may be pertinent to also ask whether the MP particles share features with processing bodies (P bodies), which play a key role in the sRNA-mediated translational inhibition in animals.41 Although P bodies and RNA transport particles are distinct in mammalian neurons, they interact by docking42 and may also fuse, as indicated by observations in Drosophila.43
Thus, the observation of mobile MP particles in leading front cells of spreading TMV infection sites opens the window for exciting new scientific questions and experimental approaches to reveal the cellular mechanisms that control and function in the transport of RNA, intercellular communication, plant development and viral disease.
Abbreviations
- MP
movement protein
- TMV
Tobacco mosaic virus
- PD
plasmodesmata
- MT
microtubules
- IB
inclusion bodies
- VRCs
viral replication complexes
- RNP
ribonucleoprotein
- vRNA
viral RNA
- ER
endoplasmic reticulum
- MF
microfilaments
- GFP
green fluorescent protein
- RFP
red fluorescent protein
- sRNA
small RNA
- siRNA
small interfering RNA
- miRNA
micro RNA
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7253
References
- 1.Deom CM, Oliver MJ, Beachy RN. The 30-kilodalton gene product of Tobacco mosaic virus potentiates virus movement. Science. 1987;237:384–389. doi: 10.1126/science.237.4813.389. [DOI] [PubMed] [Google Scholar]
- 2.Tomenius K, Clapham D, Meshi T. Localization by immunogold cytochemistry of the virus-coded 30 K protein in plasmodesmata of leaves infected with Tobacco mosaic virus. Virology. 1987;160:363–371. doi: 10.1016/0042-6822(87)90007-9. [DOI] [PubMed] [Google Scholar]
- 3.Wolf S, Deom CM, Beachy RN, Lucas WJ. Movement protein of Tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science. 1989;246:377–379. doi: 10.1126/science.246.4928.377. [DOI] [PubMed] [Google Scholar]
- 4.Citovsky V, Knorr D, Schuster G, Zambryski P. The P30 movement protein of Tobacco mosaic virus is a single-stranded nucleic acid binding protein. Cell. 1990;60:637–647. doi: 10.1016/0092-8674(90)90667-4. [DOI] [PubMed] [Google Scholar]
- 5.Brill LM, Nunn RS, Kahn TW, Yeager M, Beachy RN. Recombinant Tobacco mosaic virus movement protein is an RNA-binding, a-helical membrane protein. Proc Natl Acad Sci USA. 2000;97:7112–7117. doi: 10.1073/pnas.130187897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiki M, Kawakami S, Kim RW, Beachy RN. Domains of Tobacco mosaic virus movement protein essential for its membrane association. J Gen Virol. 2006;87:2699–2707. doi: 10.1099/vir.0.81936-0. [DOI] [PubMed] [Google Scholar]
- 7.Heinlein M, Epel BL, Padgett HS, Beachy RN. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science. 1995;270:1983–1985. doi: 10.1126/science.270.5244.1983. [DOI] [PubMed] [Google Scholar]
- 8.Heinlein M, Padgett HS, Gens JS, Pickard BG, Casper SJ, Epel BL, et al. Changing patterns of localization of the Tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell. 1998;10:1107–1120. doi: 10.1105/tpc.10.7.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padgett HS, Epel BL, Kahn TW, Heinlein M, Watanabe Y, Beachy RN. Distribution of tobamovirus movement protein in infected cells and implications for cell-to-cell spread of infection. Plant J. 1996;10:1079–1088. doi: 10.1046/j.1365-313x.1996.10061079.x. [DOI] [PubMed] [Google Scholar]
- 10.Gillespie T, Boevink P, Haupt S, Roberts AG, Toth R, Vantine T, et al. Functional analysis of a DNA shuffled movement protein reveals that microtubules are dispensable for the cell-to-cell movement of Tobacco mosaic virus. Plant Cell. 2002;14:1207–1222. doi: 10.1105/tpc.002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Más P, Beachy RN. Replication of Tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement in intracellular distribution of viral RNA. J Cell Biol. 1999;147:945–958. doi: 10.1083/jcb.147.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J. 1998;15:441–447. doi: 10.1046/j.1365-313x.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu J-Z, Blancaflor EB, Nelson RS. The Tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol. 2005;138:1877–1895. doi: 10.1104/pp.105.065722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawakami S, Watanabe Y, Beachy RN. Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc Natl Acad Sci USA. 2004;101:6291–6296. doi: 10.1073/pnas.0401221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding B, Turgeon R, Parthasarathy MV. Substructure of freeze-substituted plasmodesmata. Protoplasma. 1992;169:28–41. [Google Scholar]
- 16.Heinlein M, Epel BL. Macromolecular transport and signaling through plasmodesmata. Int Rev Cytol. 2004;235:93–164. doi: 10.1016/S0074-7696(04)35003-5. [DOI] [PubMed] [Google Scholar]
- 17.Guenoune-Gelbart D, Elbaum M, Sagi G, Levy A, Epel BL. Tobacco mosaic virus (TMV) replicase and movement protein function synergistically in facilitating TMV spread by lateral diffusion in the plasmodesmal desmotubule of Nicotiana benthamiana. Mol Plant Microbe Interact. 2008;21:335–345. doi: 10.1094/MPMI-21-3-0335. [DOI] [PubMed] [Google Scholar]
- 18.Runions J, Brach T, Kuhner S, Hawes C. Photoactivation of GFP reveals protein dynamics within the endoplasmic reticulum membrane. J Exp Bot. 2006;57:43–50. doi: 10.1093/jxb/eri289. [DOI] [PubMed] [Google Scholar]
- 19.Wright KM, Wood NT, Roberts AG, Chapman S, Boevink P, Mackenzie KM, et al. Targeting of tmv movement protein to plasmodesmata requires the actin/ER network; evidence from FRAP. Traffic. 2007;8:21–31. doi: 10.1111/j.1600-0854.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- 20.Boyko V, Ferralli J, Ashby J, Schellenbaum P, Heinlein M. Function of microtubules in intercellular transport of plant virus RNA. Nat Cell Biol. 2000;2:826–832. doi: 10.1038/35041072. [DOI] [PubMed] [Google Scholar]
- 21.Ashby J, Boutant E, Seemanpillai M, Groner A, Sambade A, Ritzenthaler C, et al. Tobacco mosaic virus movement protein functions as a structural microtubule-associated protein. J Virol. 2006;80:8329–8344. doi: 10.1128/JVI.00540-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arce-Johnson P, Kahn TW, Reimann-Philipp U, Rivera-Bustamente R, Beachy RN. The amount of movement protein produced in transgenic plants influences the establishment, local movement and systemic spread of infection by movement protein-deficient Tobacco mosaic virus. Mol Plant Microbe Interact. 1995;3:415–423. [Google Scholar]
- 23.Seemanpillai M, Elamawi R, Ritzenthaler C, Heinlein M. Challenging the role of microtubules in Tobacco mosaic virus movement by drug treatments is disputable. J Virol. 2006;80:6712–6715. doi: 10.1128/JVI.00453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prokhnevsky AI, Peremyslov VV, Dolja VV. Actin cytoskeleton is involved in targeting of a viral Hsp70 homolog to the cell periphery. J Virol. 2005;79:14421–14428. doi: 10.1128/JVI.79.22.14421-14428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyko V, Hu Q, Seemanpillai M, Ashby J, Heinlein M. Validation of microtubule-associated Tobacco mosaic virus RNA movement and involvement of microtubule-aligned particle trafficking. Plant J. 2007;51:589–603. doi: 10.1111/j.1365-313X.2007.03163.x. [DOI] [PubMed] [Google Scholar]
- 26.Sambade A, Brandner K, Hofmann C, Seemanpillai M, Mutterer J, Heinlein M. Transport of TMV movement protein particles associated with the targeting of RNA to plasmodesmata. Traffic. 2008;9:2073–2088. doi: 10.1111/j.1600-0854.2008.00824.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferralli J, Ashby J, Fasler M, Boyko V, Heinlein M. Disruption of microtubule organization and centrosome function by expression of Tobacco mosaic virus movement protein. J Virol. 2006;80:5807–5821. doi: 10.1128/JVI.00254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandner K, Sambade A, Boutant E, Didier P, Mely Y, Ritzenthaler C, et al. TMV movement protein interacts with GFP-tagged microtubule end-binding protein 1 (EB1) Plant Physiol. 2008;147:611–623. doi: 10.1104/pp.108.117481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waterman-Storer C, Salmon ED. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr Biol. 1998;8:798–806. doi: 10.1016/s0960-9822(98)70321-5. [DOI] [PubMed] [Google Scholar]
- 30.Vogler H, Kwon MO, Dang V, Sambade A, Fasler M, Ashby J, et al. Tobacco mosaic virus movement protein enhances the spread of RNA silencing. PLoS Pathog. 2008;4:1000038. doi: 10.1371/journal.ppat.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sossin WS, DesGroseillers L. Intracellular trafficking of RNA in neurons. Traffic. 2006;7:1581–1589. doi: 10.1111/j.1600-0854.2006.00500.x. [DOI] [PubMed] [Google Scholar]
- 32.Kiebler MA, Bassell GJ. Neuronal RNA granules: movers and makers. Neuron. 2006;51:685–690. doi: 10.1016/j.neuron.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Knowles RB, Sabry JH, Martone ME, Deerinck TF, Ellisman MH, Bassel GJ, et al. Translocation of RNA granules in living neurons. J Neurosci. 1996;16:7812–7820. doi: 10.1523/JNEUROSCI.16-24-07812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St Johnson D. The intracellular localization of messenger RNAs. Cell. 1995;81:161–170. doi: 10.1016/0092-8674(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 35.Palacios IM, St Johnston D. Getting the message across: the intracellular localization of mRNAs in higher eukaryotes. Annu Rev Cell Dev Biol. 2001;17:569–614. doi: 10.1146/annurev.cellbio.17.1.569. [DOI] [PubMed] [Google Scholar]
- 36.Deshler JO, Highett MI, Schnapp BJ. Localization of Xenopus Vg1 mRNA by Vera protein and the endoplasmic reticulum. Science. 1997;276:1128–1131. doi: 10.1126/science.276.5315.1128. [DOI] [PubMed] [Google Scholar]
- 37.Schmid M, Jaedicke A, Du TG, Jansen RP. Coordination of endoplasmic reticulum and mRNA localization to the yeast bud. Curr Biol. 2006;16:1538–1543. doi: 10.1016/j.cub.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Karpova OV, Ivanov KI, Rodionova P, Dorokhov YL, Atabekov JG. Nontranslatability and dissimilar behavior in plants and protoplasts of viral RNA and movement protein complexes formed in vitro. Virology. 1997;230:11–21. doi: 10.1006/viro.1997.8472. [DOI] [PubMed] [Google Scholar]
- 39.Karpova OV, Rodionova NP, Ivanov KI, Kozlovsky SV, Dorokhov YL, Atabekov JG. Phosphorylation of Tobacco mosaic virus movement protein abolishes its translation repressing ability. Virology. 1999;261:20–24. doi: 10.1006/viro.1999.9842. [DOI] [PubMed] [Google Scholar]
- 40.Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, et al. Widespread translational inhibition by plant miRNAs and siRNAs. Science. 2008;320:1185–1190. doi: 10.1126/science.1159151. [DOI] [PubMed] [Google Scholar]
- 41.Pontes O, Pikaard CS. siRNA and miRNA processing: new functions for Cajal bodies. Curr Opin Genet Dev. 2008;18:197–203. doi: 10.1016/j.gde.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeitelhofer M, Karra D, Macchi P, Tolino M, Thomas S, Schwarz M, et al. Dynamic interaction between P-bodies and transport ribonucleoprotein particles in dendrites of mature hippocampal neurons. J Neurosci. 2008;28:7555–7562. doi: 10.1523/JNEUROSCI.0104-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, et al. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52:997–1009. doi: 10.1016/j.neuron.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]