Abstract
Photoreceptors exhibit complex regulation of many aspects of growth and development, including developmental-, spatial- and temporal-specific photoregulatory responses. Such diverse regulation has been noted for all major classes of photoreceptors in plants, including red/far-red (R/FR) absorbing phytochromes and blue/UV-A (B/UV-A) light-absorbing cryptochromes and phototropins. However, the most insight into spatiotemporal responses has been reported for phytochromes both at the physiological and, more recently, at the molecular levels. Through tissue-specific degradation of the phytochrome chromophore, my laboratory recently demonstrated that phytochromes exhibit light-dependent, spatiotemporal control over de-etiolation responses in Arabidopsis thaliana. Mesophyll-localized phytochrome A (phyA) controls numerous far-red high irradiance responses (FR-HIR) in Arabidopsis. Meristem- and/or leaf primordia-localized phytochromes are involved in the regulation of leaf development. In this addendum, I provide additional novel evidence for spatial-specific, blue-light-dependent signaling roles of phytochromes.
Key words: de-etiolation, photomorphogenesis, photoreception, photoreceptor, phytochrome, red light, far-red light, blue light
Introduction
Phytochromes are plant photoreceptors that perceive maximally in the red and far-red regions of the visible spectrum and control many aspects of plant growth and development throughout the life cycle of plants. Phytochromes regulate early responses, including seed germination and seedling de-etiolation, as well as advanced stages of the life cycle such as the induction of flowering and senescence (reviewed in refs. 1–3). Phytochromes are composed of two components—an apoprotein (encoded by a family of genes) and a linear tetrapyrrole chromophore (a single molecular species named phytochromobilin). In recent years, a great deal has been learned about how phytochromes operate at the molecular level to regulate gene expression intracellularly in response to light.4,5 This gene expression response involves light-dependent movement of phytochromes from the cytosol into the nucleus of cells.
Spatial-Specific Plant Photoreceptor Responses
The roles of localized pools of phytochromes in the regulation of distinct light-dependent responses have long been recognized at the physiological level (reviewed in refs. 3, 6 and 7). However, only recently has molecular evidence for such spatial-specific light responses been reported.8–11 Enhancer trap-induced expression of PHYB in phyB-deficient background showed that mesophyll-localized phyB regulates the inhibition of hypocotyl elongation.10 PhyB also regulates an intertissue signal between the mesophyll and vascular bundles through suppressing the expression of flowering regulator FLOWERING LOCUS T (FT) as a part of the phyB-dependent control of flowering time.10 Tissue-specific expression of CRY2 in a cry2-deficient background similarly led to insights into the areas of action of cry2 in the regulation of flowering.8 Analyses of these lines indicate that in the cotyledon, cry2 exhibits cell-autonomous control over the expression of FT in its regulation of flowering time.8
In recent work, we investigated the regulatory roles of spatial-specific pools of phytochrome, particularly during de-etiolation, in Arabidopsis.11 These studies depended upon site-specific enzymatic depletion of the phytochrome chromophore through transgenic expression of a gene encoding biliverdin reductase (BVR) in plants.11 As has been shown in prior studies, expression of BVR in transgenic plants results in depletion of the phytochrome chromophore through reduction of biliverdin, a key substrate in the biosynthesis of the phytochrome chromophore.12–14 In our recent study, we selectively expressed the BVR gene using spatial-specific promoters. Analyses of the resulting transgenic lines provided novel molecular evidence for the regulatory roles of localized pools of phytochrome in specific aspects of photomorphogenesis.11
In our examination of BVR lines exhibiting meristematic and/or leaf primoridia-specific phytochrome deficiencies, we observed photoperiod-specific defects in leaf development such that MERI5::pBVR lines exhibited larger rosette diameters due to an increase in size of individual leaves.11 For mesophyll-specific depletion of phytochromes in CAB3::pBVR lines, we noted unique defects in FR-HIR responses, including the inhibition of hypocotyl elongation, opening of cotyledons, suppression of negative gravitropism, and the FR-dependent induction of anthocyanin accumulation.11 As all responses in FR are controlled primarily by phyA,15 we concluded that mesophyll-specific phyA regulates a suite of FR-HIR responses, including the control of an intercellular signal between cotyledons and hypocotyls that regulates the inhibition of hypocotyl elongation. The attribution of this mesophyll-specific response to phyA is in agreement with prior findings demonstrating that phyA is the phytochrome responsible for the FR-dependent inhibition of hypoctyl elongation.16–18 Of note was the novel finding that hypocotyl-localized phyA may contribute to the regulation of cell elongation in Arabidopsis.11
Spatial-Specific Regulation of Blue-Light-Dependent Responses by Phytochrome A
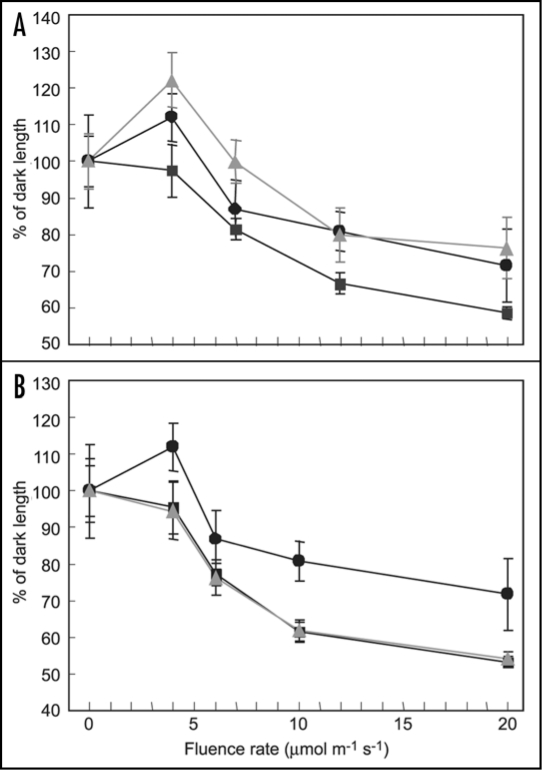
In addition to R and FR light, the wavelengths of light that phytochromes absorb maximally, phytochromes are capable of absorbing light in the blue region of the visible spectrum19 and plants exhibit phytochrome-dependent, blue light responses. Specifically, phyA has a role in inhibiting hypocotyl elongation in continuous blue (Bc) light.15,18,20–22 It has been shown that phyA has additional roles under Bc, including activation of chloroplast transcription in mature leaves under B.23 Furthermore, it was reported recently that phyA has a major role in the early induction of gene expression for GUN4 and CHLH in Bc light.24 Results from prior studies demonstrated that 35S::pBVR lines exhibit elongated hypocotyls under Bc light.12 This latter observation, taken together with the identified roles for phyA in regulating plant responses to B illumination, led to an investigation of the impact of Bc light on seedling development in BVR lines. I compared the responses of BVR lines exhibiting spatial-specific phytochrome deficiencies to WT and 35S::pBVR plant lines. As shown in Figure 1, mesophyll-specific inactivation of photoactive phytochromes results in elongated hypocotyls under increasing fluences of Bc. The pattern observed for a representative CAB3::pBVR3 line, CAB3::pBVR2, is similar in degree to that observed for the constitutively BVR-expressing 35S::pBVR3 line (Fig. 1A). In contrast, there was no difference observed between the Bc-dependent inhibition of hypocotyl elongation for No-O WT and MERI5::pBVR1 plant lines (Fig. 1B). Thus, mesophyll-specific phytochromes regulate hypocotyl elongation under increasing fluences of blue light. Given earlier findings discussed above that phyA has a role in inhibiting hypocotyl elongation under blue illumination, the phenotype observed may be associated with the action of mesophyll-localized phyA. However, a physiological role for phyC in this process cannot be ruled out as a prior report described a modulatory effect of phyC on the inhibition of hypocotyl elongation under Bc illumination.25 Nevertheless, this BL-dependent regulation likely involves a phytochrome-dependent inter-organ signal between cotyledons and hypocotyls, similar to that proposed to be active in phyA-dependent regulation of the inhibition of hypocotyl elongation under FRc.11
Figure 1.
Fluence response curve for hypocotyl length of wild-type and transgenic BVR seedlings. CAB3::pBVR2 and MERI5::pBVR1 lines are compared with No-O WT and 35S::pBVR3 seedlings grown at 20°C on Phytablend medium containing 1% sucrose for 7 d under continuous blue light of various fluence rates. Data points represent mean (+SD) of hypocotyl lengths measured for 10 to 25 seedlings in each of three independent experiments, and correspond to No-O WT (■), 35S::pBVR3 (●) and CAB3::pBVR2/MERI5::pBVR1 (▲, gray).
Conclusions
Spatiotemporal regulation of plant growth and development is not a new phenomenon (reviewed in ref. 7). However, emerging tools and biochemical approaches are allowing researchers to gain new insight into the molecular bases of spatial-specific environmental responses. Recent reports and new data presented here provide breakthroughs in our understanding of the sites of photoperception and sub-organismal locations of pools of photoreceptors that control distinct aspects of light-dependent growth and development. These results and other recent reports provide new molecular information about a subset of photoreceptor-dependent responses that have long been recognized at the physiological level based on experiments using selective irradiation and dissected plant parts.7 Additional investigations using emergent technologies and the development of additional methods for probing spatiotemporal responses will allow continued progress on understanding the molecular bases of these important aspects of plant photomorphogenesis.
Acknowledgements
Research on light sensing and photomorphogenesis in plants in the author's laboratory is supported by the U.S. Department of Energy (Energy Biosciences Program, DE-FG02-91ER20021 to B.L.M.). The author would like to thank Sankalpi Warnasooriya for critically reading and commenting on the manuscript.
Abbreviations
- B
blue light
- cry
cryptochrome
- FR
far red light
- HIR
high irradiance response
- phy
phytochrome
- R
red light
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7271
References
- 1.Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annu Rev Genet. 2004;38:87–117. doi: 10.1146/annurev.genet.38.072902.092259. [DOI] [PubMed] [Google Scholar]
- 2.Franklin KA, Larner VS, Whitelam GC. The signal transducing photoreceptors of plants. Int J Dev Biol. 2005;49:653–664. doi: 10.1387/ijdb.051989kf. [DOI] [PubMed] [Google Scholar]
- 3.Josse E-M, Foreman J, Halliday KJ. Paths through the phytochrome network. Plant Cell Environ. 2008;31:667–678. doi: 10.1111/j.1365-3040.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 4.Jiao YL, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8:217–230. doi: 10.1038/nrg2049. [DOI] [PubMed] [Google Scholar]
- 5.Kevei E, Schafer E, Nagy F. Light-regulated nucleo-cytoplasmic partitioning of phytochromes. J Exp Bot. 2007;58:3113–3124. doi: 10.1093/jxb/erm145. [DOI] [PubMed] [Google Scholar]
- 6.Bou-Torrent J, Roig-Villanova I, Martinez-Garcia JF. Light signaling: back to space. Trends Plant Sci. 2008;13:108–114. doi: 10.1016/j.tplants.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery BL. Right place, right time: Spatiotemporal light regulation of plant growth and development. Plant Signal Behav. 2008;3 doi: 10.4161/psb.3.12.6857. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo M, Mochizuki N, Suzuki T, Nagatani A. CRYPTOCHROME2 in vascular bundles regulates flowering in Arabidopsis. Plant Cell. 2007;19:84–93. doi: 10.1105/tpc.106.048157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endo M, Nagatani A. Flowering regulation by tissue specific functions of photoreceptors. Plant Signal Behav. 2008;3:47–48. doi: 10.4161/psb.3.1.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo M, Nakamura S, Araki T, Mochizuki N, Nagatani A. Phytochrome B in the mesophyll delays flowering by suppressing FLOWERING LOCUS T expression in Arabidopsis vascular bundles. Plant Cell. 2005;17:1941–1952. doi: 10.1105/tpc.105.032342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warnasooriya SN, Montgomery BL. Detection of spatial-specific phytochrome responses using targeted expression of biliverdin reductase in Arabidopsis thaliana. Plant Physiol. 2008 doi: 10.1104/pp.108.127050. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagarias DM, Crepeau MW, Maines MD, Lagarias JC. Regulation of photomorphogenesis by expression of mammalian biliverdin reductase in transgenic Arabidopsis plants. Plant Cell. 1997;9:675–688. doi: 10.1105/tpc.9.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery BL, Franklin KA, Terry MJ, Thomas B, Jackson SD, Crepeau MW, Lagarias JC. Biliverdin reductase-induced phytochrome chromophore deficiency in transgenic tobacco. Plant Physiol. 2001;125:266–277. doi: 10.1104/pp.125.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery BL, Yeh KC, Crepeau MW, Lagarias JC. Modification of distinct aspects of photomorphogenesis via targeted expression of mammalian biliverdin reductase in transgenic Arabidopsis plants. Plant Physiol. 1999;121:629–639. doi: 10.1104/pp.121.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neff MM, Chory J. Genetic interactions between phytochrome A, phytochrome B and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998;118:27–35. doi: 10.1104/pp.118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parks BM, Quail PH. hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell. 1993;5:39–48. doi: 10.1105/tpc.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler WL, Hendricks SB, Siegelman HW. Action spectra of phytochrome in vitro. Photochem Photobiol. 1964;3:521–528. [Google Scholar]
- 20.Duek PD, Fankhauser C. HFR1, a putative bHLH transcription factor, mediates both phytochrome A and cryptochrome signalling. Plant J. 2003;34:827–836. doi: 10.1046/j.1365-313x.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- 21.Poppe C, Sweere U, Drumm-Herrel H, Schafer E. The blue light receptor cryptochrome 1 can act independently of phytochrome A and B in Arabidopsis thaliana. Plant J. 1998;16:465–471. doi: 10.1046/j.1365-313x.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 22.Weller JL, Beauchamp N, Kerckhoffs LH, Platten JD, Reid JB. Interaction of phytochromes A and B in the control of de-etiolation and flowering in pea. Plant J. 2001;26:283–294. doi: 10.1046/j.1365-313x.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 23.Chun L, Kawakami A, Christopher DA. Phytochrome A mediates blue light and UV-Adependent chloroplast gene transcription in green leaves. Plant Physiol. 2001;125:1957–1966. doi: 10.1104/pp.125.4.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephenson PG, Terry MJ. Light signalling pathways regulating the Mg-chelatase branchpoint of chlorophyll synthesis during de-etiolation in Arabidopsis thaliana. Photochem Photobiol Sci. 2008;7:1243–1252. doi: 10.1039/b802596g. [DOI] [PubMed] [Google Scholar]
- 25.Franklin KA, Davis SJ, Stoddart WM, Vierstra RD, Whitelam GC. Mutant analyses define multiple roles for phytochrome C in Arabidopsis photomorphogenesis. Plant Cell. 2003;15:1981–1989. doi: 10.1105/tpc.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]