Abstract
High-lipid oat is a potential oil crop. Chemical and microscopical analyses have shown that the major part of the grain lipids are stored in the endosperm. While oil bodies are intact in the aleurone layer, scutellum and embryo, they have less associated proteins (oleosins) and undergo fusion in the starchy endosperm. In this report, we document the distribution of lipids in the endosperm microscopically. Underneath the aleurone layer, lipids are most abundant in the subaleurone cells and in the endosperm cells in the vicinity of the scutellum and embryo. Thus the major areas of oil storage are close to the living tissues of the grain, the sites of enzyme production in connection with germination and mobilization. The documentation of cellular structural changes, and implication of the fused state of oil bodies, during germination, remains to be elucidated.
Key words: oat, oil, aleurone, scutellum, embryo, endosperm
Oat (Avena sativa L.) is a cereal crop that accumulates up to 18% of lipids.1 Most of the lipids are stored in the endosperm tissues of the grain.2–4 This has been also demonstrated using fluorescence microscopy,1,5 light microscopy as well as scanning and transmission electron microscopy.4,6 White et al.,7 on the other hand, interpreted their transmission electron microscopy pictures as if indicating scarcity or absence of lipids beneath the subaleurone layer. Applying different microscopical approaches, it has been shown that oil bodies appear as distinct entities in the embryonic axis and the aleurone layer, and that they loose their integrity and fuse with each other into large masses in the rest of the endosperm during grain development.6 The fusion of oil bodies in the starchy endosperm is probably correlated with the reduced amount of the protecting oil-body associated proteins (oleosins) documented in this tissue.6
The aim of this report is to present microscopical data on the distribution of oil throughout the oat endosperm, not shown in Heneen et al.6 The structural observations were made on the high-lipid oat cultivar Matilda with 10.3% lipids.6 Sectioned sectors of grains at early and late developmental stages, 14 and 40 days after anthesis, were stained with Sudan Black B or Toluidine Blue O.6 The low magnifications of whole sectors of the grain and higher magnifications of defined sites (Fig. 1) provide information on the distribution and appearance of lipids throughout the grain. A further step would be simultaneous visualization and quantification of lipids, as has been elegantly demonstrated in developing barley grains and soybean seeds in vivo, applying frequency-selected magnetic resonance imaging.8
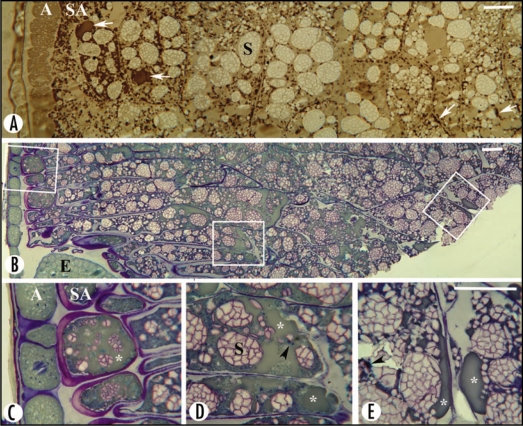
Figure 1.
Sectors and enlarged framed areas of sectioned oat grains at early (A) and late (B–E) stages of development, showing the appearance and distribution of oil. (A) Sudan black B staining. Oil bodies are grey in the aleurone layer (A) and brown/black in the rest of the endosperm. They occur mainly as discrete entities and sporadically as fused aggregates (arrows) in all endosperm cells, and are most abundant in the subaleurone cells (SA). Starch granules/aggregates (S) are lightly stained. Protein bodies are tiny and brownish in colour (not indicated). (B–E) Toluidine blue staining of a grain sector comprising part of the scutellum and the embryo (E). The magnified framed areas display subaleurone cells (C), endosperm cells close to the scutellum-embryo (D) and inner endosperm cells (E), in which the oil bodies have fused into a matrix that stains green (asterisks), with embedded darkly stained proteins (arrowheads) and blue-contoured starch granules/aggregates (S). Oil is more predominant in the subaleurone cells and in endosperm cells close to the scutellum-embryo region than in the inner endosperm cells. Bar = 30 µm.
At the early developmental stage of oat grains (Fig. 1A), lipids were less densely stained in the aleurone layer than in the rest of the endosperm, inferring tissue-specific differences. Oil was not evenly distributed in the starchy endosperm. It was most frequent in the subaleurone cells, and decreased in cells of middle and inner endosperm, in accordance with earlier findings.5 This was valid for the discrete oil bodies observed at early stages of development (Fig. 1A), and for the fused oil masses or what appeared as an oil matrix that prevailed at late stages of development (Fig. 1B–E). In addition, endosperm cells in the vicinity of the embryonic axis contained higher amounts of oil (Fig. 1D) when compared with cells more distant or more inward from the embryo (Fig. 1E). Thus higher concentrations of lipids are stored close to the aleurone layer and the embryo, which are the living parts of the grain. Accordingly, endosperm cells with the highest amounts of oil will be first exposed to hydrolases and lipases in connection with germination and mobilization. In malting barley and other germinating cereal grains, it has been established that features indicating cellular activity, and production of hydrolases and amylases, are initiated in the scutellum followed by the aleurone layer, and that degradation of endosperm cells starts in the vicinity of the scutellum.9–11 Intact oil bodies thought essential for optimal germination conditions are challenged by their fused state in storage tissues of the oat endosperm. The implications of their fusion for germination and end use quality remain to be determined. Breeding efforts to develop high-lipid oats, if successful, would place oat among the oil crops that are of potential use in future chemical and health-related industries.12
Corresponding to the oil bodies in plants are the lipid droplets in animal cells, considered as possible multifunctional organelles, involved in signalling and trafficking of molecules.13,14 Whether oil bodies in plants have additional functions other than just storage of lipids remains to be discovered. Yet, it has been suggested that the term lipid droplets could be used universally.13
Acknowledgements
We thank Gunnel Karlsson for skilful technical assistance with the preparation of sections for microscopy.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7313
References
- 1.Peterson DM, Woods DF. Composition and structure of high-oil oat. J Cereal Sci. 1997;26:121–128. [Google Scholar]
- 2.Youngs VL, Püskülcü M, Smith RR. Oat lipids I. Composition and distribution of lipid components in two oat cultivars. Cereal Chem. 1977;54:803–812. [Google Scholar]
- 3.Price PB, Parsons J. Distribution of lipids in embryonic axis, bran-endosperm, and hull fractions of hulless barley and hulless oat grain. J Agric Food Chem. 1979;27:813–815. [Google Scholar]
- 4.Banas A, Debski H, Banas W, Heneen WK, Dahlqvist A, Bafor M, Gummeson P-O, Marttila S, Ekman Å, Carlsson AS, Stymne S. Lipids in grain tissues of oat (Avena sativa): differences in content, time of deposition and fatty acid composition. J Exp Bot. 2007;58:2463–2470. doi: 10.1093/jxb/erm125. [DOI] [PubMed] [Google Scholar]
- 5.Fulcher RG. Morphological and chemical organization of the oat kernel. In: Webster FH, editor. Oats: Chemistry and Technology. St. Paul, MN, USA: Am Assoc Cereal Chem; 1986. pp. 47–74. [Google Scholar]
- 6.Heneen WK, Karlsson G, Brismar K, Gummeson P-O, Marttila S, Leonova S, Carlsson AS, Bafor M, Banas A, Mattsson B, Debski H, Stymne S. Fusion of oil bodies in endosperm of oat grains. Planta. 2008;228:589–599. doi: 10.1007/s00425-008-0761-x. [DOI] [PubMed] [Google Scholar]
- 7.White DA, Fisk ID, Gray DA. Characterisation of oat (Avena sativa L.) oil bodies and intrinsically associated E-vitamers. J Cereal Sci. 2006;43:244–249. [Google Scholar]
- 8.Neuberger T, Sreenivasulu N, Rokitta M, Rolletschek H, Göbel C, Rutten T, Radchuk V, Feussner I, Wobus U, Jakob P, Webb A, Borisjuk L. Quantitative imaging of oil storage in developing crop seeds. Plant Biotech J. 2008;6:31–45. doi: 10.1111/j.1467-7652.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons GC. On the sequential determination of α-amylase transport and cell wall breakdown in germinating seeds of Hordeum vulgare. Carlsberg Res Commun. 1980;45:177–184. [Google Scholar]
- 10.Gram NH. The ultrastructure of germinating barley seeds I. Changes in the scutellum and the aleurone layer in normal barley. Carlsberg Res Commun. 1982;47:143–162. [Google Scholar]
- 11.Jensen SA, Heltved F. Visualization of enzyme activity in germinating cereal seeds using a lipase sensitive fluorochrome. Carlsberg Res Commun. 1982;47:297–303. [Google Scholar]
- 12.Dyr JM, Stymne S, Green AG, Carlsson AS. High-value oils from plants. Plant J. 2008;54:640–655. doi: 10.1111/j.1365-313X.2008.03430.x. [DOI] [PubMed] [Google Scholar]
- 13.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nature Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 14.Welte MA. Proteins under new management: lipid droplets deliver. Trends Cell Biol. 2007;17:363–369. doi: 10.1016/j.tcb.2007.06.004. [DOI] [PubMed] [Google Scholar]