Abstract
The plant signals strigolactones activate seed germination of the parasitic weeds (Striga and Orobanche), growth of arbuscular mycorrhizal (AM) fungi and have recently been described as a new class of plant hormones that inhibit shoot branching. In AM fungi, the synthetic strigolactone analogue GR24 rapidly stimulates mitochondrial metabolism (within minutes) and biogenesis (within one hour). New gene expression, more active nuclear division and cell proliferation occur later (within days). By using pharmacological approaches to inhibit the mitochondrial ATP synthesis, various steps of the respiratory chain and the mitochondrial protein translation, we further describe the mechanisms underlying the mitochondrial response to GR24. We show with SHAM and KCN inhibition treatments that the respiratory chain of Gigaspora rosea is branched and includes an alternative oxydase. The two electron transports can be used for GR24 activation of hyphal branching but only the alternative one is used for spore germination. By using the inhibitors Oligomycin, Rotenone, Antimycine A and KCN, we show that indirect (proton pumping) and direct inhibition of ATP synthase does not completely abolish the activation of hyphal branching by GR24. However, hyphal branching was totally inhibited with the suppression of mitochondrial biogenesis, confirming the essential role played by mitochondria to amplify the strigolactone response of AM fungi.
Key words: strigolactones, mitochondria, catabolism, lipids, AOX
Strigolactones are plant signal molecules initially characterized as seed germination stimulants of the parasitic weeds Orobanche and Striga.1 Recent findings have revealed that natural strigolactones or the synthetic strigolactone analogue GR24 can also elicit hyphal branching of arbuscular mycorrhizal (AM) fungi, a cellular response typically observed when these fungi grow close to a living root.2
Moreover when plants are affected in strigolactone production their capacity to be mycorrhized is strongly reduced.3,4 An additional role of strigolactones, as possible novel plant hormones inhibiting shoot branching, has recently been discovered.4,5 The cellular modes of action of strigolactones on seeds of parasitic weeds and on plant axillary buds are not known. In previous studies we showed, on the AM fungus Gigaspora rosea, that GR24 elicits a fast (within minutes) synthesis and utilisation of NADH by mitochondria. This boost of mitochondrial activity was correlated, after one hour of GR24 treatment, with an enhancement of cellular ATP production. This enhancement of oxydative metabolism precedes active mitochondrial proliferation.6,7 Taken together, these results suggested that mitochondrial activation is a key event required for switching the fungus to a pre-symbiotic stage, characterized by a more active nuclear proliferation, an upregulation of several genes and an activated cell proliferation (the so called branching response).6
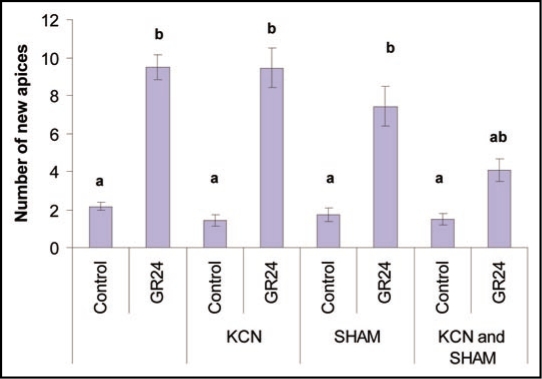
In order to clarify the role of mitochondria in the GR24 response, we carried out a series of pharmacological experiments where different steps of the oxidative phosphorylation were specifically inhibited. We first inhibited mitochondrial ATP synthesis with oligomycin, an inhibitor of ATP synthase subunit Fo, or with a cocktail of Rotenone, Antimycine A and KCN (inhibitors that block the proton pumping from the matrix to the inter membrane space) (Fig. 1). Surprisingly only 36.7% to 50% of the hyphal branching response was suppressed. Hyphal elongation (not shown) was not affected by the treatment with the cocktail of the 3 last inhibitors suggesting that catabolic pathways other than the mitochondrial oxidative phosphorylation produced enough ATP to sustain normal hyphal activity. The same hypothesis can be proposed to explain that the GR24 branching response was only partially reduced. As triglycerides are the main carbon storage form in AM fungi,8 lipid catabolism, in the absence of oxidative phosphorylation, can only provide ATP (actually GTP) during the oxidation of acetyl CoA in the citric acid cycle. Under normal condition we can postulate that most ATP production associated with lipid degradation is sustained in mitochondria by the respiratory chain. Thus, if the citric acid cycle still works in mitochondria despite the inhibition of the respiratory chain, it implies that mitochondria are able (i) to achieve the reoxydation of NADH (produced during the β-oxidation of fatty acids and the citric acid cycle) in the absence of a fonctional complex I and (ii) to transfer electrons from NADH to O2 in the absence of a functional cytochrome c oxidase. This would be possible if the respiratory chain of G. rosea contained an alternative NADH dehydrogenase and alternative oxidase (AOX) as already described in other fungi.9 To investigate the hypothesis that G. rosea possesses a mitochondrial alternative oxydase, single and double inhibitions of the AOX and the cytochrome c oxidase were carried out. Only a simultaneous inhibition of AOX and cytochrome c oxidase by SHAM and KCN, respectively, led to a reduction of 57% of the branching response intensity (Fig. 2). This result strongly suggests that G. rosea mitochondria have a ramified respiratory chain and that the role of O2 as a final electron acceptor is important to get the GR24 branching response. In contrast with the GR24 branching response, germination of G. rosea spores could be completely inhibited with a SHAM treatment, suggesting that spore germination is exclusively dependent on the activity of AOX (data not shown), as it is for seed germination of Orobanche sp.10
Figure 1.
Inhibition of the ATP synthase or loss of proton gradient is not sufficient to totally suppress the hyphal branching activation by GR24. Spores of Gigaspora rosea were grown for six days on solid M medium in the dark at 28°C under 2% CO2 and then treated with 10−7 M GR24 alone or simultaneously with the various inhibitors. A 28 µM oligomycin treatment was applied to specifically inhibit ATP synthesis. H+ pumping activities by the respiratory complexes I, III and IV were inhibited by treating the germinated spores with a mix of 20 µM Rotenone, 19 µM antimycin A and 1.5 mM KCN. After 24 h, the number of new apices formed was counted. Values are the mean of three biological replicates. Thirty spores were used in each experimental condition. Means with the same letter are not significantly different (ANOVA test and Tuckey post-hoc Analysis). Data were computed with the R software.
Figure 2.
Partial inhibition of the hyphal branching activation by GR24 requires conjugated action of KCN and SHAM. Spores of Gigaspora rosea were grown for six days on solid M medium in the dark at 28°C under 2% CO2 and then treated with 10−7 M GR24 and with 1.5 mM KCN, 5 mM SHAM or both. After 24 h, the number of new hyphal apices formed was counted. Values are the mean of three biological replicates. Twenty to 30 spores were used in each treatment. Means with the same letter are not significantly different (ANOVA test and Tuckey post-hoc Analysis). Data were computed with the R software.
The other catabolic pathway that could produce ATP other than in mitochondria is the glycolytic pathway. AM fungi, like plants, can also degrade fatty acids through the glyoxylate cycle and produce succinate.11 Succinate can thereafter serve as a biosynthetic intermediate and be later oxidized through the glycolytic pathway. Another, more likely, possibility is that the catabolism of glycogen, an additional form of carbon storage in AM fungi,12 is also stimulated by strigolactones.
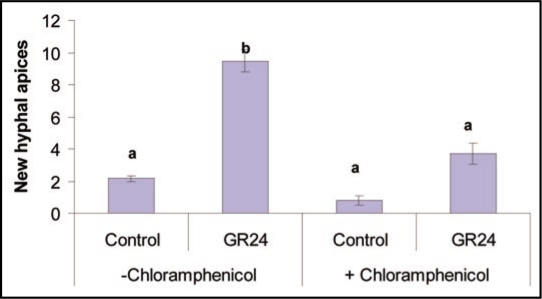
Whereas we can speculate that strigolactones do not directly stimulate oxidative phosphorylation (otherwise inhibition of oxidative phosphorylation would have had more impact on GR24-induced hyphal branching), mitochondrial complete oxidation process seems necessary to get a maximal hyphal branching response. This was further demonstrated by an inhibition experiment of mitochondrial biogenesis. This biogenesis requires mitochondrial protein biosynthesis,13,14 a process that can be specifically inhibited with chloramphenicol. Preliminary assays showed that after a chloramphenicol treatment, mitochondria of GR24 treated hyphae did not change their shape or movements like normally observed in GR24-treated hyphae7 (data not shown). This indicates that, at this concentration, chloramphenicol was able to inhibit mitochondrial biogenesis in the fungus. When germinated spores of G. rosea were treated with both chloramphenicol and GR24 for 24 h, GR24-induced branching was decreased by 46.8%, whereas no significant effect was visible on control hyphae not treated with GR24 (Fig. 3).
Figure 3.
Inhibition of the synthesis of mitochondrial proteins in Gigaspora rosea affects hyphal branching activation by GR24. Spores of Gigaspora rosea were grown for six days on solid M medium in the dark at 28°C under 2% CO2 and then treated with 10−7 M GR24, 1 mg/ml chloramphenicol or both. After 24 h, the number of new hyphal apices formed was counted. Values are the mean of three biological replicates. Ten to 20 spores were used in each treatment. Means with the same letter are not significantly different (ANOVA test and Tuckey post-hoc Analysis). Data were computed with the R software.
In conclusion, our data suggest that Gigaspora rosea possesses a ramified respiratory chain with an alternative NADH dehydrogenase and an AOX, the activity of the latter being essential for spore germination. They also suggest that, in the activation of hyphal branching by strigolactones in AM fungi, upstream catabolic processes, such as the β-oxidation of fatty acids (followed by the citric acid cycle) or glycogen degradation (followed by glycolysis), could be activated before oxidative phosphorylation. This is in agreement with a recent report15 which showed that lipid catabolism of Glomus intraradices is enhanced in response to root exudates. The stimulation of mitochondrial biogenesis that occurs later would be a consequence of these first metabolic activations and would then largely amplify them.
Similar mechanisms of catabolic activation could be involved in the strigolactone induction of seed germination of parasitic weeds by strigolactones.16 In contrast, it is more difficult to imagine that strigolactone inhibition of plant lateral bud outgrowth also requires an activation of ATP synthesis. In order to evaluate to which extent they are conserved, the mode of action of strigolactones on the three biological systems need to be further investigated.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7419
References
- 1.Cook CE, Whichard LP, Turner B, Wall ME, Egley GH. Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science. 1966;154:1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Roldan V, Girard D, Bécard G, Puech-Pages V. Strigolactones: promising plants signals. Plant Signal Behav. 2007;2:163–164. doi: 10.4161/psb.2.3.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Bécard G, Beveridge CA, Rameau C, Rochange SF. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 5.Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 6.Besserer A, Bécard G, Jauneau A, Roux C, Séjalon-Delmas N. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol. 2008;148:402–413. doi: 10.1104/pp.108.121400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besserer A, Puech-Pages V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais JC, Roux C, Bécard G, Séjalon-Delmas N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006;4:226. doi: 10.1371/journal.pbio.0040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sancholle M, Dalpé Y, Grandmougin-Ferjani A. Lipids of mycorrhizae. The mycota IX. 2001:63–92. [Google Scholar]
- 9.Joseph-Horne T, Hollomon DW, Wood PM. Fungal respiration: a fusion of standard and alternative components. Biochem Biophys Acta. 2001;1504:179–195. doi: 10.1016/s0005-2728(00)00251-6. [DOI] [PubMed] [Google Scholar]
- 10.Bar Nun N, Plakhine D, Joel DM, Mayer AM. Changes in the activity of the alternative oxidase in Orobanche seeds during conditioning and their possible physiological function. Phytochemistry. 2003;64:235–241. doi: 10.1016/s0031-9422(03)00165-1. [DOI] [PubMed] [Google Scholar]
- 11.Lammers PJ, Jun J, Abubaker J, Arreola R, Gopalan A, Bago B, Hernandez-Sebastia C, Allen JW, Douds DD, Pfeffer PE, Shachar-Hill Y. The glyoxylate cycle in an arbuscular mycorrhizal fungus. Carbon flux and gene expression. Plant Physiol. 2001;127:1287–1298. [PMC free article] [PubMed] [Google Scholar]
- 12.Bago B, Pfeffer PE, Douds DDJ, Brouillette J, Bécard G, Shachar-Hill Y. Carbon metabolism in spores of the arbuscular mycorrhizal fungus Glomus intraradices as revealed by nuclear magnetic resonance spectroscopy. Plant Physiol. 1999;121:263–272. doi: 10.1104/pp.121.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkie D. Mitochondrial biogenesis: inhibitors of mitochondrial protein synthesis. Mol Cell Biochem. 1977;14:97–100. doi: 10.1007/BF01734171. [DOI] [PubMed] [Google Scholar]
- 14.Forde BG, Oliver RJ, Leaver CJ. In Vitro study of mitochondrial protein synthesis during mitochondrial biogenesis in excised plant storage tissue. Plant Physiol. 1979;63:67–73. doi: 10.1104/pp.63.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bücking H, Abubaker J, Govindarajulu M, Tala M, Pfeffer P, Nagahashi G, Lammers P, Shachar-Hill Y. Root exudates stimulate the uptake and metabolism of organic carbon in germinating spores of Glomus intraradices. New Phytol. 2008;180:684–695. doi: 10.1111/j.1469-8137.2008.02590.x. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Nun N, Mayer AM. Composition and changes in storage compounds in Orobanche aegyptiaca seeds during preconditioning. Isr J Plant Sci. 2002;50:277–279. [Google Scholar]